Background
With fifty-thousand catheter ablations being performed in Europe every year electrophysiology (EP) is a rapidly growing practice (1) The constant development of cardiovascular imaging and clinical research have expanded the range of available tests. A number of different imaging technologies with overlapping capabilities are now available in the EP field. These imaging studies are carried out ahead of the electrophysiological study in dedicated scanners and then transferred into the EP lab in order to be integrated within the EP study setup (2). Several recent imaging techniques also allow 3D reconstruction of the heart. An understanding of these different imaging technologies is indispensable and it is currently recommended that physicians be trained in several imaging modalities (3) and physiological simulations (4).
I - Modalities
Fluoroscopy, intracardiac echogram (ICE), computed tomography (CT), rotational angiography (RA) and cardiac magnetic resonance (CMR) are presented as their integration into the EP lab.
A) Fluoroscopy
Availability: Fluoroscopy is readily available at a low cost all over the world. It is the standard imaging technique for EP procedures.
User’s manual: Interventionists acquire the expertise necessary to obtaining proper orientation and detail from looking at only a single 2D picture.
Side effects: Fluoroscopy can cause serious side effects for both the investigator and the patient. Being in the direct ionising beam, the patient is exposed to maximum levels while the operator is has the highest exposure to scattered radiation.
Use: Radiofrequency (RF) ablation procedure produces 350–2000 times the levels of radiation of a chest X-ray for the patient. However this is normally a once in a lifetime situation (5).
Safety: The potential to induce malignancies by fluoroscopic exposure in interventional procedures is relatively low, although repeated exposures might become an issue in this regard.
Closest adherence to the ALARA (as low as reasonably achievable) principle is the best protection against radiation injury. Regarding this topic the ESC recently published a practical compendium to reduce radiation dose for patients and staff (6). For all these reasons the use of non-fluoroscopic navigational mapping systems to guide the ablation procedure are recommended.
B) Computed tomography
Technology: Dedicated computer systems generate a 3D image of the heart from a large series of 2D fluoroscopic images. The latest, with high resolution and fast speed multi-slice techniques, allow shortened 5 minutes procedure times.
Uses: CT is useful to map anatomy for procedure planning (as variation of PVs, Figure 1) and to assess complications.
Images have high spatial resolution and can show extra-cardiac structures in 3D with excellent reproducibility.
Application of contrast in a timely fashion allows the exact delineation of the cardiac anatomy in the diastole.
Limitations: CT high-resolution images necessitate a radiation dose up to 12 mSv (equivalent to 100-600 chest X-rays) (7). Recent innovations to the technique, however, have substantially reduced exposure to radiation (8). Other potential limitations are the low functional information (offered instead by the CMR) and the lack of temporal resolution.
Figure 1: Example of LA segmentation from a CT scan.
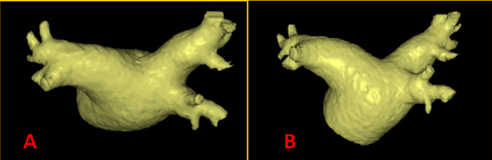
In the superior view (A) and a PA projection (B) a left common trunk of the pulmonary veins is evident.
C) Cardiac magnetic resonance
Technology: In contrast to CT imaging, CMR imaging uses non-ionising radiation and magnetic fields to align the nuclear magnetisation of (mostly) hydrogen atoms in the water content of the body. This allows the blood to act as the contrast agent.
Uses: CMR can help in the planning of an intervention and identifying of potential complications and potentially, in risk stratification before the ablation procedure, based on the location and extent of atrial fibrosis (9). However, mapping of fibrosis by MRI remains an investigational technique at present.
Limitations: A limitation of the CMR technology is the exclusion of patients with implanted devices (10). However, this is becoming less of a problem with the increasing number of MRI-compatible devices (11).
D) Rotational angiography
Technology: A very recent development in 3D imaging in the same procedure as the invasive HP study has been introduced by means of rotational angiography (RA). Using a timed injection of contrast in order to fill the target chamber (e.g. the left atrium), the X-arm is rotated around the patient to acquire multiple 2D pictures. Using dedicated software, these 2D pictures are reconstructed to provide 3D images of the given area of interest (12).
Uses: The ease of use and accurate 3D representation of the left atrium make 3D RA a reasonable alternative to conventional 3D electroanatomical mapping systems, however, without advanced mapping functions (13).
E) Echocardiography
Uses: Echocardiography provides useful information regarding anatomy and possible presence of underlying abnormalities. It is often performed in the routine workup of patients, preceding the ablation procedure.
To exclude the presence of a left atrial appendage thrombus, transesophageal echocardiography (TEE) is recommended (14) in all patients undergoing a catheter ablation procedure for atrial fibrillation (AF) (15). In addition, TEE may facilitate the transseptal puncture and is especially helpful in distorted anatomy. Invasive intracardiacechocardiography (ICE) Also may be used to direct transseptal puncture and manipulation of catheters (16).
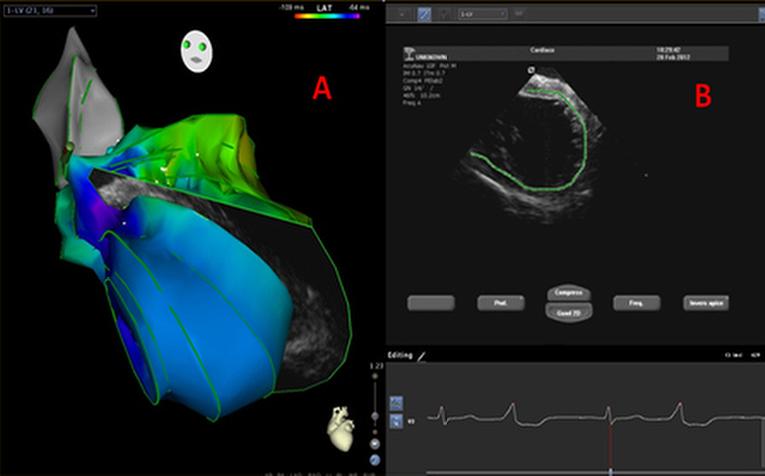
Technology: A new technology, CARTOSOUND® (Biosense Webster, Diamond Bar, CA, USA), enables a 3D reconstruction of the cardiac chambers derived from the endocardial surfaces, which is apparent on ICE, including catheter location and orientation (Figure 2). Choosing the corresponding imaging plane during ablation could potentially allow for visualising lesion formation in real time and guiding a substrate-based ablation (17).
Figure 2: Example of CARTOSOUND 3D reconstruction of the LV endocardial surfaces (A) from ICE (B)
F) 3D electroanatomical mapping systems
Technology: A major step forward in clinical electrophysiology has been the introduction of 3D electroanatomic mapping (EAM) systems, integrating three important functionalities, namely 1) non-fluoroscopic localisation of electrophysiological catheters in three-dimensional (3D) space; 2) analysis and 3D display of activation sequences computed from local or calculated electrograms (Figure 3), and 3D display of electrogram voltage ('scar tissue'); and 3) integration of this electroanatomic information with non-invasive images of the heart (18) Two such systems are currently available in most of the EP-laboratories: the magnetic-based CARTO® system (Biosense Webster), and the impedance-based EnSiteTM NavXTM system (St. Jude Medical, St. Paul, MN, USA).
Figure 3: Example an activation map during EAM of a typical flutter (NavX system)
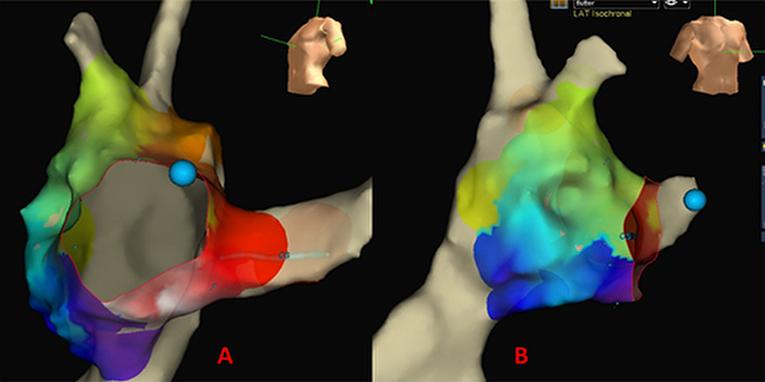
Uses: EAM systems provide online electrophysiological data, and allow tracking of mapping/ablation catheters and annotation of ablation points (19).
Technology: Algorithms aimed at balancing heart shift during the breathing cycle (respiratory compensation) and manual elimination of “false spaces” and erroneous structure definition are important for the purpose of better reproducing the true cardiac anatomy. Additionally, uniform point sampling is essential to avoiding geometry distortion.
Adequate catheter contact while acquiring surface points is determined by fluoroscopic visualisation of catheter mobility in relation to cardiac motion, a discrete electrogram and in some catheters the specific icon on the 3-dimensional navigation system.
Limitations: A limitation of electro-anatomical mapping systems (EAM), however, is the use of reconstructed anatomy. Suboptimal catheter tip-to-tissue contact force during lesion delivery could reduce clinical efficacy of ablation (20).
G) Image integration
Uses: Computing a 3D image from 2D digital imaging allows for instant visualisation of the cardiac chambers. Various software programs allow these images to be combined with the 3D mapping information (image merging, Figure 4). Registration of the optimal alignment is key because misalignment could lead to a misinterpretation of the imaging information. Image integration is especially valuable when complex anatomy or unusual presentations can easily confuse the operator.
Figure 4: Example of image merging of the left atrium made by electroanatomic mapping system
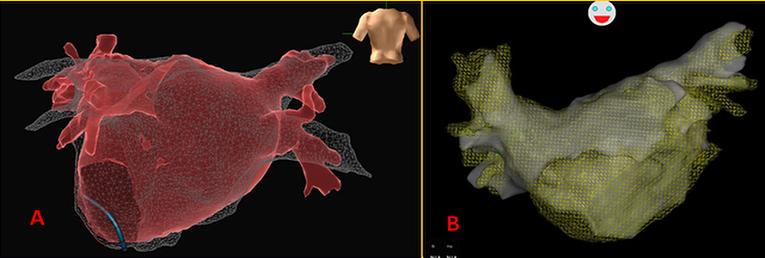
A) PA projection, EnSite NavX system; B) AP projection, CARTO system
In addition, catheter-related myocardial wall stretch occurring during EAM does not affect wall reconstruction obtained from radiological images. Localisation of surrounding structures can also avoid high-output energies while ablating in close proximity of these. In order to minimise differences between the geometry at the time of image acquisition and the ablation procedure, the intervening period should be reduced as much as possible.
Pre-procedural imaging and its image integration with the EAM should be mandatory in complicated anatomy as in the grown-up congenital heart (GUCH) population. For these patients arrhythmias remain important causes of late morbidity and mortality. Arrhythmia mechanisms vary according to the exact underlying anatomic congenital defect, method and timing of surgical repair. A proper understanding of the 3D altered anatomy is key to selecting the appropriate technologies (21).
II - Remote navigation
The need to diminish the risk to patients and the risk to physicians related to X-ray radiation should becom a priority for the future. Increased use of sophisticated remote-control technologies might offer a means to reducing such exposure. Two systems are mainly used today:
- Robotic: The remote robotic navigation system (Sensei® Robotic System; Hansen Medical, Inc., Mountain View, CA, USA) consists of a standard ablation catheter integrated into a remote steerable double sheath, a robotic arm and a workstation equipped with a tactile feedback system (22). If combined with contact force sensing, the remote robotic navigation technology can result in more efficient transmural ablation lesions, but actually the quality of the available evidence is still too low (23).
- Magnetic: Another remote technology is the magnetic navigation system (MNS) (Niobe® MNS; Stereotaxis, St Louis, MO, USA). It employs a soft ablation catheter that is manipulated by two external 0.08-0.1 Tesla magnetic fields situated on either side of the patient. The catheter tip aligns with the magnetic vector produced by the system, allowing the operator to navigate the catheter to the desired site from the control room (24). A motor drive unit performs advancement and retraction of the catheter and the remote navigation is performed with a joystick or mouse from the control room. The MNS also allows for a further reduction of radiation exposure for the patient due to the option of storing and reapplying once-visited sites and of a reduced number of required diagnostic catheters. Furthermore, the soft catheter tip makes tissue damage including perforation almost impossible (25). This has also the advantage of improving power delivery with superior flexibility and accuracy in areas that prove challenging with respect to catheter stability (26).
Conclusions
The use of imaging provides unrestricted access to the cardiac anatomy and could guide interventional cardiac procedures through high-resolution images of the heart, complex procedures made more manageable and with fewer complications. Advantages also include shorter procedure times and reduced exposure to radiation. These have led to its increasing use in the EP lab such that a clear understanding of these different imaging technologies is indispensable.



 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.