Background
The most common symptom of peripheral artery disease (PAD) in the lower extremities is intermittent claudication [1]. Patients with claudication experience reversible muscle ischemia characterized by persistent cramp-like pain and aching in the affected muscle while they walk. The symptoms severely limit both exercise performance and walking ability. As a consequence, PAD is associated with reduced physical functioning and quality of life [2].
Supervised exercise programs have been recommended as first-line therapies for the treatment of claudication in patients with PAD [3-5]. The treatment goals are: (1) to reduce limb symptoms, (2) to improve exercise capacity and prevent or lessen physical disability, and (3) to reduce the occurrence of cardiovascular events.
This article summarizes the current state of exercise therapy in patients with PAD and intermittent claudication.
Intermittent claudication
Intermittent claudication is a cardinal symptom in patients with PAD. The classic manifestation is muscle discomfort (patients may complain of muscle fatigue, aching, or persistent cramp-like pain) in the lower limb reproducibly produced by exercise and relieved by short-term rest.
PAD is caused by stenotic or occlusive atherosclerotic lesions in the major arteries supplying the lower extremities. Patients with intermittent claudication have normal blood flow at rest. With exercise, stenotic/occlusive lesions in the arterial supply of the leg muscles limit the increase in blood flow, causing a mismatch between oxygen supply and the metabolic demand of the muscle [1]. Patients with PAD shift more quickly to anaerobic metabolism, a less efficient means of energy production, during exercise, and have elevated lactate levels even at rest [6]. Acquired metabolic abnormalities of the muscles of the lower extremity also contribute to the reduced exercise performance and capacity of patients with PAD [2,6].
Claudication significantly affects quality of life and is associated with severe functional impairment that can be significantly improved by exercise intervention in properly selected patients [6].
Guidelines and indications for exercise therapy
The treatment of limb symptoms and exercise limitation should initially focus on structured, supervised exercise before any attempts are made to revascularize patients with claudication. A considerable body of evidence supports the clinical benefits of a supervised exercise program in improving exercise performance and quality of life [7].
The ESC [3], AHA/ACC [4], and Trans-Atlantic Inter-Society Consensus Document on Management of Peripheral Arterial Disease (TASC II) [5] have all declared that the evidence supporting exercise therapy in the treatment of claudication is sufficiently robust to merit a Level I recommendation. Table 1 shows the recommendation and evidence levels for exercise therapy in the recently published practice guidelines of the Society for Vascular Surgery [8].
Table 1. Recommendations: Exercise therapy [8].
|
|
Grade |
Level of evidence |
|---|---|---|
|
We recommend as first-line therapy a supervised exercise program consisting of walking a minimum of three times per week (30-60 min/session) for at least 12 weeks to all suitable patients with IC. |
1 |
A |
|
We recommend home-based exercise, with a goal of at least 30 minutes of walking three to five times per week when a supervised exercise program is unavailable or for long-term benefit after a supervised exercise program is completed. |
1 |
B |
|
In patients who have undergone revascularization therapy for IC, we recommend exercise (either supervised or home based) for adjunctive functional benefits. |
1 |
B |
|
We recommend that patients with IC be followed up annually to assess compliance with lifestyle measures (smoking cessation, exercise) and medical therapies as well as to determine if there is evidence of progression in symptoms or signs of PAD. Yearly ABI testing may be of value to provide objective evidence of disease progression. |
1 |
C |
ABI: ankle-brachial index; IC: intermittent claudication; PAD: peripheral arterial disease
Several medical comorbidities, such as angina, congestive heart failure, chronic obstructive pulmonary disease, or arthritis, may preclude patient participation in exercise programs. Patients should therefore be evaluated to ensure that their comorbidities are sufficiently well controlled to allow safe participation in such a program [3-5,8]. Exercise is also contraindicated by foot ulcers and/or limb pain at rest (Fontaine class III/IV) in patients awaiting revascularization.
Exercise program
The fundamental component of training is a supervised program of treadmill exercise [5]. The exercise session begins with treadmill exercise at a speed and grade that induce claudication within 3 to 5 minutes. The patient is instructed to stop walking and rest when his or her claudication pain reaches a moderate level. When the claudication has abated, the patient resumes walking until moderate claudication discomfort recurs. This cycle of exercise and rest is repeated for at least 30 minutes in the first few sessions of the program. In subsequent visits, the speed or grade of the treadmill is increased if the patient is able to walk for 10 minutes or longer at a lower workload without reaching moderate claudication pain.
The duration and frequency of the exercise training sessions and duration of the exercise training program are important to achieve maximal benefit with training sessions: >30 minutes per session provides greater benefit than <30 minutes per session; >3 sessions per week is more effective than <3 sessions per week, and program lengths of >26 weeks are more effective than program lengths of <26 weeks [9].
Alternatives to treadmill exercise potentially consist of various forms of lower extremity exercise alone or in combination (brisk walking, bicycle ergometer, and strength training). However, the outcomes of treadmill exercise have so far been found to be superior to the outcomes of several other lower extremity exercises, namely cycling, stair climbing, and static and dynamic leg exercises [10].
While home exercise (unsupervised) programs can be modified or supplemented to improve their effectiveness, structured, supervised exercise programs generally have superior outcomes compared to unsupervised programs. A few studies have demonstrated that patients completing home-based exercise programs were able to improve both their initial claudication distance and absolute claudication distance [11,12].
Mechanisms underlying the benefits of exercise therapy
PAD is characterized by obstructions in lower extremity blood flow. However, obstruction to lower extremity blood flow does not appear to be the only determinant of functional impairment in PAD. Studies have shown evidence of an ischemia-related myopathy in calf skeletal muscle consisting of calf muscle atrophy associated with mitochondrial dysfunction and increased infiltration of fat tissue into the muscle [6]. Moreover, this condition is independent of differences in physical activity levels between individuals with vs. without PAD. Thus, the reversal of lower extremity arterial obstruction with revascularization does not reverse functional impairment as robustly as it improves the lower extremity blood flow [13]. Supervised treadmill exercise, on the other hand, improves functional performance but generally shows no accompanying effect in improving lower extremity arterial obstruction [7].
The potential biomechanical or biochemical mechanisms underlying the benefits of exercise therapy are exercise-induced angiogenesis, enhanced nitric oxide-dependent vasodilatation of the microcirculation, improved hemorheology, reduced vascular inflammation, improved glucose and fatty acid metabolism in skeletal muscle, improved muscle bioenergetics and oxidative stress, improved peripheral nerve function, and so on [2,6,14].
In summary (Figure 1), the mechanisms underlying the response to exercise training include improvements in:
- Blood perfusion
- Muscle metabolism and mitochondrial function
- Peripheral nerve function
- Walking efficiency
Figure 1. Schema of the potential mechanisms underlying the benefits of exercise therapy on walking performance in peripheral artery disease.
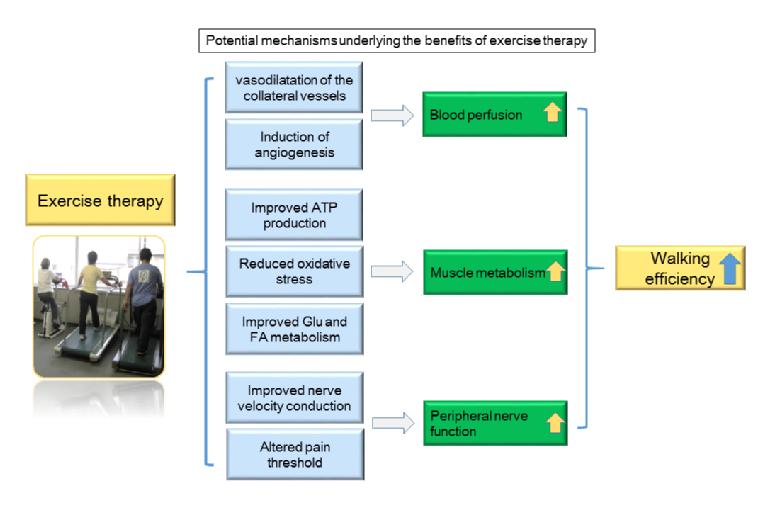
FA: fatty acid; Glu: glucose
Exercise vs. endovascular therapy
Limited controlled data are available comparing revascularisation with exercise training for intermittent claudication. The efficacy of supervised exercise training compared with endovascular revascularisation as an initial treatment strategy for patients with claudication and aortoiliac disease was evaluated in the CLEVER study. [15,16] The trial randomized 111 patients with moderate-to-severe claudication due to aortoiliac occlusive disease to one of three treatments: optimal medical care, optimal medical care plus supervised exercise, or optimal medical care plus stent revascularisation. The study subjects achieved significant improvements in peak walking time and claudication onset time when treated with either supervised exercise training or stenting, compared with optimal medical care alone. This benefit was durable for at least 18 months. [16] Although patients with supra-inguinal arterial lesions can be considered for revascularisation without initially undergoing an extensive regimen of medical therapy with exercise training, the benefit of exercise therapy was equal to that of an invasive stent strategy and was maintained for a full year after completion of the supervised training phase with help from a telephone-based counseling system.
A meta-analysis of currently available data [17] suggests that endovascular therapy alone in patients with intermittent claudication provides no improvement in outcome over exercise training alone and that endovascular therapy plus supervised exercise brings about the most favorable outcomes in function.
Beyond improving walking performance
Less exercise tolerance leads to lower survival outcome in patients with cardiovascular disease. [18] Poor walking performance and inactivity were associated with cardiovascular and all-cause mortality in patients with PAD. [2,6]
Exercise and increased physical activity ameliorate the chronic inflammation and the multiple risk factors, as well as exercise capacity in subjects with atherosclerotic vascular disease. [6] Accumulating evidence from clinical trials has shown the positive impact of exercise-based cardiac rehabilitation on quality of life and cardiovascular events and mortality in patients with coronary artery disease. [19]
It remains unclear, however, whether exercise intervention improves mortality in patients with PAD. One retrospective cohort study from Japan showed that a 12-week supervised exercise training program reduced overall cardiovascular mortality by 52% and morbidity by 30% within a maximum of 13 years of follow-up. [20] The results from that study demonstrate the promise of exercise training for secondary prevention in PAD. A larger prospective interventional study will be necessary to confirm the observation.
Other considerations
The major problem of intermittent claudication is that it is accompanied by a strong increased risk. Lipid lowering, smoking cessation and other lifestyle and medical changes are essential steps to decrease risk and improve prognosis. Both physicians and patients need to understand the importance of this: it is not by exercise alone that we can control the total cardiovascular risk. [3-5,8]
Conclusion
Exercise therapy has multiple benefits via multiple mechanisms in PAD patients with intermittent claudication, including reduced limb symptoms, improved functional capacity, and reduced systemic cardiovascular risk. Exercise training also shows promise as a therapy for improving functional impairment in asymptomatic patients with PAD. [2]
Exercise, even unsupervised and low-intensity exercise, should be recommended and encouraged for all patients with PAD without contraindications. However, several factors, such as medical comorbidities, referral and insurance systems, and patient willingness to participate and adhere, limit the use of supervised exercise programs.



 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.