Background
Whereas pulmonary hypertension is a pathophysiological state defined by an increased in mean pulmonary arterial pressure ≥ 25 mm Hg at rest as assessed by right heart catheterisation, pulmonary arterial hypertension, on the other hand, is a distinct clinical group of uncommon conditions characterised only by 1) the presence of pre-capillary pulmonary hypertension and 2) the absence of other causes of pulmonary hypertension (left heart disease, lung disease, chronic thromboembolic pulmonary hypertension or other rare diseases).
Pulmonary arterial hypertension is associated with a high mortality rate (1) thus, improving treatment outcome is especially important. A strategic objective is early detection and this can be achieved through early diagnosis of 1) pulmonary vascular disease and 2) right ventricular dysfunction; however problems in so doing are that 1) the rise in resting pulmonary arterial pressure is a late marker of the remodeling process occurring in the pulmonary arterial wall and 2) symptoms of right ventricular failure do not occur at rest until the latter stages of pulmonary vascular disease (2).
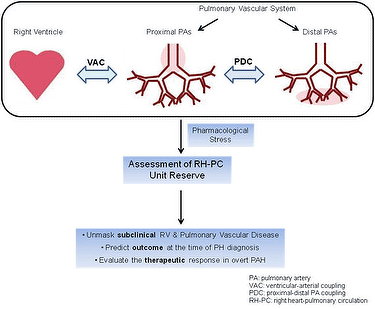
We have migrated our focus from the pulmonary vasculature or the right ventricular function to the right heart-pulmonary circulation unit (RH-PCU), with special interest in evaluating the dynamic response of the RH-PCU to either exercise or pharmacological stress (3).
Here reviewed is the concept of ventricular-vascular reserve and its potential clinical value in pulmonary artery hypertension.
I - Stress testing of the right heart-pulmonary circulation unit in healthy subjects
Response of the pulmonary vasculature: the ability to accommodate the flow increase
Pulmonary circulation is unique in its ability to increase available capillary surface for gas exchange using a recruitable vascular reserve. Indeed, pulmonary circulation can accommodate cardiac output at low arterial pressure even during maximum exercise stress (4): the relative modest increment in mean pulmonary arterial pressure (mPAP) relative to cardiac output (CO) during exercise or pharmacological testing is attributable to passive recruitment and distension of a normally compliant pulmonary circulation with active flow-mediated vasodilatation, decreasing pulmonary vascular resistance (PVR) and total pulmonary resistance (TPR) (5). The greater pulmonary vascular distensibility (α) and increase in the capillary blood component of lung diffusing capacity during exercise, the higher the maximal aerobic capacity (6).
A feasible technique for stress testing of the pulmonary vasculature is to assess the mPAP-CO relationships. Invasive and non-invasive studies alike have shown that slope of linearised mPAP-CO relationships should not exceed 3 mm Hg/L/min (7). Additionally, we reported that passive increase of CO (dobutamine stress in the Trendelenburg position) was associated with a decrease in arterial stiffness (elastic modulus, EM); the preserved buffering function could explain the unchanged pulmonary vascular capacitance (8).
In accordance, Guihaire et al showed very recently in normal piglets that dobutamine stress did not change arterial elastance (Ea) (9). The normal reserve of conduit and buffering functions is thought to maintain the proximal-distal pulmonary arterial coupling during stress (10).
Response of the right ventricle: the ability to increase cardiac output
Cardiac output of the RV increases during submaximal exercise. The CO increase composed of a heart rate and right heart stroke volume (SV) increase (11). We also reported that pharmacological stress determines a 56% increase in heart rate (chronotropic reserve) and a 20% increase in stroke volume (SV, systolic reserve) (8). Measuring RV contractile response to stress, Guihaire et al reported a significant increase of RV maximal elastance (Emax) during dobutamine stress, improving the Emax/Ea ratio from 1.24 to 2.14. The Emax/Ea ratios obtained in control subjects at rest were 1.9 ± 0.2 (12) and 1.5 ± 0.3 (13). Finally, Sharma et al obtained significant augmentations in both tricuspid annular plane systolic excursion (TAPSE, 25%) and tricuspid annulus systolic myocardial velocity (S', 90%) during peak dobutamine stress (20 mg/kg/min) (14).
Assessment of RH-PCU reserve using pharmacological stress is theoretically preferable
Although dynamic exercise remains the most physiologic stressor in that patients symptoms and signs are replicated, the assessment of RH-PCU reserve using pharmacological stress is an appealing alternative to exercise stress due to theoretical advantages:
- Exercise hemodynamic studies bring less information regarding pulmonary vascular function since the slope of mPAP-CO relationships is affected by exercise-induced pulmonary vasoconstriction and changes in left atrial pressure (15).
- A purely passive increase of pulmonary blood flow achieved by low-dose dobutamine might better analyse the pulmonary vascular pressure-flow characteristics for different types of PH.
Non-invasive echocardiography: only by trained ecosonographers
Non-invasive echocardiography is not considered totally reliable for the study of pulmonary circulation (16,17) due to an excessive proportion of false positives and false negatives in the detection of PH (18), as well as a suboptimal rate in the detection of tricuspid regurgitation jets. Exercise echocardiography is technically demanding and requires high expertise to the rapid acquisition of high quality images in tachypnoeic patients. However, in experienced and trained ecosonographers non-invasive assessment of RV contractile reserve and mPAP-CO relationships are feasible with dobutamine stress with respect to exercise stress.
II - Stress testing of the right heart-pulmonary circulation unit in PAH patients
Evaluation of right heart-pulmonary circulation unit reserve : functional parameters are best
The most identified prognostic indices in PAH have derived from direct measurements of RV function - echocardiography and cardiac magnetic resonance imaging - or surrogate indicators of RV dysfunction such as right atrial pressure and cardiac index (19). Functional parameters (NYHA class, 6 min walking distance) and, in particular, cardiopulmonary exercise testing variables at the time of diagnosis have a superior prognostic significance to most standard resting haemodynamic parameters. Added to that fact, all exercise variables which have prognostic significance when determined at baseline, retain their prognostic relevance after treatment, and some, such as change of cardiac index (CI) from baseline upon treatment are an important tool for assessing disease severity and prognosis (20,21).
The cardiovascular reserve function is emerging a strong predictor of outcome in heart failure and cardiovascular disease. Stressors (exercise or pharmacological) in patients with PAH test the ability of the diseased right heart to increase its output (former) and of the remodeled pulmonary arterial system to accommodate to the flow increase (latter). From a physiological point of view the former (ability to increase the cardiac output) evaluates the RV systolic reserve and the latter (ability to accomodate the flow increase) assesses the vascular reserve.
Ventricular and vascular reserve: suggested during dobutamine stress for early diagnosis
Some PAH patients with RV dysfunction at rest do much better in their exercise capacity, functional class and quality of life than others with the same impaired RV pump function at rest. It is proposed that these might differ in their RV or vascular reserve. Methods and indexes to assess cardiovascular reserve function need to be standardised. Ventricular and vascular reserve metrics can be estimated as the normalised change in ventricular and vascular function during stress when compared with rest.
Two studies have recently studied the systolic RV reserve by surrogate indices and their relationship with functional state and survival in patients with PAH.
- In an invasive study, Blumberg et al. observed a close relationship between exercise cardiac index and peak VO2 in patients with PH. They also showed that among hemodynamic variables, only the exercise cardiac index, and the slope of the mPAP-CO relationship were significant prognostic indicators (22).
- In a non-invasive study, Grünig et al. proposed that exercise-induced systolic PAP increases may indicate RV contractile reserve in patients with severe PAH or inoperable chronic thromboembolic pulmonary hypertension (23). They observed that patients who revealed a high exercise-induced systolic PAP increase (> median value of 30 mm Hg), had a significantly higher mean six-minute walking distance and mean peak VO2, lower NT-proBNP and right atria area, and better 1-, 3-, and 4-year survival rates.
- Very recently, one experimental work and three clinical studies have assessed RV systolic reserve and vascular reserve in PH and evaluated the relationship between cardiovascular reserve with exercise capacity, RV-arterial coupling and prognosis.
- In a piglet model of chronic PH, Guihaire et al reported that while stroke volume index (SVI) and CI were not significantly decreased at rest in the PH group, contractile reserve measured by peak or relative change in SVI or CI were significantly impaired. They found an impaired ventricular-arterial coupling in the PH group with a strong relationship between ventricular-arterial coupling (Emax/Ea) and RV contractile reserve (delta Emax).
- In the clinical scenario, the non-invasive assessment of mPAP-CO relationships and the pulmonary vascular distensibility coefficient α ( dobutamine stress echocardiography,up to 20mg/kg/min) showed that patients with PAH had an average dobutamine-induced mPAP-CO slope of 5.1 ± 2.5 compared to 1.1 ± 0.7 mm Hg/L/min in healthy controls, with a markedly reduced dobutamine-induced distensibility coefficient α. Furthermore, in patients with PAH, lower functional class status was associated with lower dobutamine-induced mPAP-CO slope. The same group reported that the RV contractile reserve (TAPSE and S') was markedly attenuated in the PAH patients compared with controls, even in a subgroup of PAH patients with preserved resting RV systolic function. Finally, RV contractile reserve correlates with exercise capacity suggesting the potential clinical value beyond resting measurements (24).
- Our group evaluated simultaneously the RV systolic reserve (TAPSE, S', myocardial acceleration during isovolumic contraction, IVA) and the pulmonary vascular reserve (arterial stiffness) using low-dose of dobutamine (10mg/kg/min) plus a 30° Trendelenburg position and its impacts on the outcome during a two year follow-up in patients with PAH. We demonstrated that global reserve of the RV-PCU was impaired in PAH patients, showing the higher increase in delta EM, higher mPAP-CO ratio, with a negative change in delta SV and a positive change in delta PVR and TPR. While delta EM correlated with delta mPAP, delta S' and delta IVA correlated with CO, therefore the stress response of mPAP and CO would be in relation with the pulmonary vascular reserve and systolic RV reserve, respectively. The lower recruitable reserve of the RV-PCU was significantly related to a worse hemodynamic response to stress testing showing a higher dobutamine-induced mPAP-CO slope, and it could be associated with poor clinical outcome (Fig 1).
Conclusion
Patients with pulmonary arterial hypertension exhibit markedly diminished right ventricular contractile reserve and arterial pulmonary vascular reserve. Abnormal stress responses occur even in patients with a relatively preserved resting hemodynamic at rest. There is evidence that impairment of cardiovascular stress response correlates with exercise capacity and prognosis, suggesting the potential clinical value of measures of cardiovascular reserve beyond resting measurements.
Taking into account that hemodynamic changes (mPAP-CO relationships) generated by dobutamine and exercise stress may not be interchangeable, and that exercise stress can induce spurious increase in the mPAP-CO slope, we propose the dobutamine stress to assess right ventricular-pulmonary unit reserve.
Further validation studies are required to assess the potential utility of measuring the cardiovascular reserve during dobutamine stress for early diagnosis, prognosis, and monitoring of therapeutic response in patients with suspected or established pulmonary arterial hypertension before implementation into clinical practice.


 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.