Background
Tako-tsubo cardiomyopathy (TTC), also referred to as stress cardiomyopathy, apical ballooning syndrome or “broken heart syndrome” is an acute catecholamine-induced myocardial inflammation occurring mainly in aging women after severe stress. Acute development of segmental - usually periapical - left ventricular systolic dysfunction occurs almost exclusively, and is often precipitated by exposure to severe acute emotional stress. In a cohort study of 136 patients, 96% were female and about 90% were over 50 years of age. Clinically, it typically mimics acute myocardial infarction (MI), with acute onset of severe chest pain, dyspnoea, and ECG changes typical of MI - often with S-T segment elevation initially. Evaluation of left ventricular function by echocardiography usually reveals a periapical zone of akinesis or hypokinesis. Several investigations have shown that left ventricular (LV) wall motion may return to normal within a few weeks and symptoms may subside within a few days, yet severe complications such as LV free wall rupture, pulmonary oedema, heart failure, arrhythmias, dynamic LV outflow tract obstruction, hypotension and even death may occur (1-4).
Exclusion of ischaemia and demonstration of extensive myocardial inflammation
There is evidence that clinical consequence of TTC is myocardial inflammation initiated by “pulse” exposure to catecholamines in susceptible myocardium. Available mechanistic data from a rat model suggest that the main effects of catecholamines are mediated by β2-aderenoceptor stimulation (5), and we have preliminary evidence to suggest that the coupling of such receptors with synthesis of nitric oxide may also be important (6). Associated with inflammatory activation and nitrosative stress there is probably also myocardial energetic impairment (7). Initial reports suggested that this was a relative rarity, and did not really delineate pathogenesis. Under such circumstances, it became effectively a diagnosis requiring exclusion of ischaemia (8). It is now clear that coronary disease may theoretically co-exist, and that diagnosis ultimately rests on the demonstration of extensive myocardial inflammation yet from a clinical point of view, differentiation from acute MI remains potentially difficult. In table 1, the therapeutic implications of differentiation with AMI/ischaemia are listed.
Table 1: Therapeutic implications of differentiation with AMI/ischaemia.
| Common problems | AMI only | TTC only |
|---|---|---|
| 1. Hypotension/ Shock (however, rare in Non-Q-wave AMI) |
1. Re-infarction | 1. Torsade de pointes |
| 2. Risk of LV mural thrombosis (mainly in TTC) | 2. Long-term systolic LV dysfunction | 2. LV outflow track obstruction |
| 3. Ongoing chest pain | 3. Aggravation with catecholamine administration | |
| 4. Aggravation with nitric oxyde donors |
Mayo clinic
The Mayo clinic requires the presence of all four criteria:
- Regional wall motion abnormalities (mid-LV and/or apical transient hypokinesis, akinesis, or dyskinesis);
- Absence of obstructive coronary artery disease or acute plaque rupture on angiography;
- New ST-segment elevation and/or T-wave inversion; or modest serum cardiac troponin elevation;
- Absence of pheochromocytoma or myocarditis (9).
However, we note that these updated criteria (2008) do not mention:
- Rationale for excluding patients with a history of pheochromocytoma (where an adrenal gland releases hormones that cause persistant or episodic high blood pressure) or myocarditis (10). Possible presence of incidental coronary disease of coronary artery disease is also not included.
- Selection bias from catherisation - The condition has usually been recognised, particularly in the early series of cases, as a result of cardiac catheterisation in patients presenting with acute chest pain in which initial diagnosis was acute coronary syndrome. These reports largely related to patients presenting with initial S-T segment elevation on ECG, and therefore triaged to emergency cardiac catheterisation, however more recent studies indicate that only 40% of presentations include initial S-T segment elevation (11, 12).
- Single or multivessel coronary spasm as a possible underlying disorder - the distribution of hypokinesis rarely accords with the course of a single coronary artery.
Very elderly, frail patients presenting with prolonged chest pain, especially without S-T elevation, may not undergo cardiac catheterisation, and therefore the diagnosis may easily be missed. All these reasons make it essential to establish consistent diagnostic criteria.
General considerations
From study results not involving cardiac catheterisation which had thus far dominated, two differences with acute myocardial ischaemia/infarction have been put forward:
- Limited release of markers of myocardial injury: Low levels of troponin (Tn) and creatine kinase (CK) are noted as indeed most patients have no long-term evidence of myocardial infarction on cardiovascular magnetic resonance (CMR) scanning.
- Myocardial stunning: No infarction combined with rapid improvement of regional LV wall motion suggest that it may represent a form of reversible chemical injury analogous to myocardial “stunning”. Indeed, a number of recent studies have revealed evidence of regional intracardiac inflammatory changes (from biopsy studies) (14) and oedema (from CMR studies) after acute attacks (15).
How clinicians should differentiate it from Non-Q-wave acute myocardial infarction (NQAMI)
1 - Clinical presentation
Approached according to the mode of presentation and duration of symptoms
- Sudden emotional or physical stress: It is often triggered by sudden emotional or physical stress brought on by individually or communally stressful circumstances. The critical issue is that of the probability of precipitation by the stimuli concerned, which is unknown.
- Relationship with acute cerebral infarction: It is a well-recognised complication of both cerebral infarction and haemorrhage (16), but embolic cerebral infarction is also a potential complication (17).
- Stressors: Some patients bring up no stressors however, the majority experienced at least one recent severe stress event and 9 out of 10 were female (18).
- ACS symptoms: Clinical presentation usually mimics the symptoms of acute coronary syndrome with variable degrees of chest pain at rest in 50 to 60% of patients, and/or dyspnoea.
- Hypotension and shock are frequent early complications: the mechanism(s) responsible for haemodynamic impairment are probably combinations of impaired left ventricular contractility, together with inadequate tachycardic response and in some cases BNP-mediated vasodilatation (19).
2 - Bloodwork
The following tests do not enable a clear distinction between TTC and AMI patients.
- Cathecolamines - Laboratory findings are similar to those of acute coronary syndrome. Compared to MI patients, a significant elevation of catecholamine levels on admission has been reported by Wittstein et al however, no significant difference in catecholamine levels with MI patients has been documented in other studies (20). This may be explained by the differences in time-course from time of the first symptom presentation, to the time that the actual blood sample is taken.
- Elevated biomarkers - Cardiac biomarkers such as Tn, CK, and CK MB are slightly elevated in most cases (21). Substantial increases in BNP and NT-pro BNP have been found in several studies (22).
3 - ECG changes
Individual changes in electrocardiogram vary, but may be similar to changes in MI patients with ST elevation, T-wave inversion, and pathological Q-waves (23).
- Heterogeneous S-T and T wave changes - In a systemic review by Pilgrim & Wyss (21), ST segmental elevation was documented in more than 70% of patients, particularly in the precordial leads. However, in another study of 105 patients, only 34.2% had ST segmental elevation on ECG (11). Subsequently, T-wave inversion has been found to be very common. Furthermore, a prolonged QT interval has often been recognised (24) and torsades de pointes may occur as a result (25,26). Several attempts have been made to distinguish associated ECG changes from those of AMI patients with anterior infarction with S-T elevation due to left anterior descending (LAD) occlusion. In all, ST-segment elevation and progressive T-wave inversion on ECG are not reliable criteria to differentiate these conditions (27). Nevertheless, TTC tends to be associated with “anatomically heterogeneous” S-T and T wave changes, suggesting involvement of more than one area of coronary perfusion (Figure 1).
Figure 1: Typical pattern of QT prolongation and “multi-regional” T wave inversion in Takotsubo cardiomyopathy.
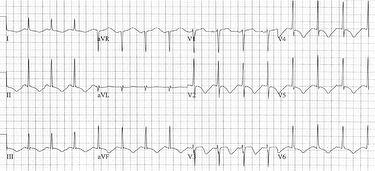
4 - Echocardiography
LV apical ballooning - Changes on echocardiography often show apical and/or midventricular akinesis/hypokinesis and basal hyperkinesis, distorting the shape of the LV, and showing “LV apical ballooning”. Approximately one third of patients also have apical right ventricular hypokinesis (28). The contractile dysfunction of the LV results in a lowered LVEF. Moderate to severe LV systolic/diastolic dysfunction is common but can be absent. Although the wall thickness in the akinetic segments may be reduced in the acute phase, the characteristics of these thinned regions have been reported to be different from the hyperechogenic fibrosis signals of a transmural MI. More importantly, the segmental rather than global wall motion abnormalities, which are not associated with any single coronary artery territory, could be used to distinguish it from AMI (29). Using a speckle tracking echocardiographic methods to quantitate regional LV systolic function, Mansencal et al. (30) reported that there were significant differences in strain, strain rate and systolic peak velocities between acute and chronic phases. Furthermore, in the acute phase, systolic dysfunction was circular with similar velocity among basal, mid ventricle, and apical segments and was different from that of patients with LAD coronary artery obstruction.
5 - Cardiac catheterisation/coronary angiography
Diagnosis is most commonly made by invasive coronary angiography and is rarely the initial diagnosis at hospital admission. In most cases, no significant coronary artery stenoses are found. Left ventriculography is a useful method to diagnose and classify forms, typically demonstrating the distinctive LV shape - similar to a Japanese octopus pot with a round bottom and a narrow neck. Approximately 30% of cases exhibit predominantly mid-ventricular hypokinesis, while there is also a rare basal or “inverted” form (31).
Regarding haemodynamics during the acute stages, these are common findings: 1) systemic hypotension or shock (19), 2) mild elevation of pulmonary capillary wedge pressure - although pulmonary oedema is rare (22). Occasionally, patients have haemodynamic evidence of intraventricular pressure gradients and/or severe mitral valve regurgitation. Due to the need for cardiac catheterisation (using the Mayo clinic criteria), the diagnostic process is subject to potential selection bias, as this investigation is more likely to be performed in patients with severe symptoms.
6 - Cardiovascular magnetic resonance
Cardiovascular magnetic resonance (CMR) allows assessment of cardiac function and LV regional wall motion abnormalities with high resolution. Using T2-weighted CMR, periapical oedema is observed in most patients (32). However, careful T2 quantitation reveals oedema throughout the LV (15). Although delayed enhancement is not usually seen in patients with TTC on contrast-enhanced CMR, it has been reported in some cases (due to increase in extracellular matrix content, confirmed with collagen-1 staining) (32).The detection of marked plasma BNP/NT-proBNP elevation as well as a positive troponin T concentration in female patients with prior emotional or physical trauma and presence of ECG showing “multi-regional” changes and no ST segment elevation is suggestive of TTC. Assessments of regional LV wall motion using either echocardiography or coronary angiography followed by detection of myocardial oedema without infarction on CMR (with late gadolinium enhancement and T2-weighted imaging) are the steps which complete the diagnosis.
Treatment
Therapeutics for TTC have not been firmly established yet they are to:
- Monitor for arrhythmias for at least 2 days
- Anti-coagulate with heparin to reduce risk of mural thrombosis
- Avoid administration of catecholamines.
- Avoid organic nitrates since there is evidence that TTC may include nitrosative stress (33)
- Use ACE inhibitor therapy which may reduce the risk of recurrence (34) in the long-term according to a meta-analysis.
However, therapeutics for 1) management of shock and 2) accelerating recovery of LV function are still awaiting clinical trial.
Conclusions
TTC is a common differential diagnosis of AMI, irrespective of presence of S-T elevation. Differentiation is important because treatment is also different: while patients presenting with S-T elevation need to undergo cardiac catheterisation as the first diagnostic step, in practice this functions in parallel with clinical assessment, detection of elevated BNP/NT-proBNP levels, ECG performance and echocardiography as initial diagnostic tests and should be followed by the demonstration of extensive myocardial oedema on CMR.


 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.