Background
Minimally Invasive Heart valve Sugery (MIHVS) is commonly performed through a right mini thoracotomy (1 2,3), a parasternal incision - adjacent to the sternum - or a hemi-sternotomy. Available evidence shows comparable or better results compared to the conventional surgical approach (4, 5, 6, 7) which involves a full median sternotomy. Antegrade ascending aorta cannulation is adopted whenever possible (usually for aortic valve surgery).
Another commonly used option is femoral artery cannulation with retrograde arterial perfusion. Although there has been debate over whether retrograde arterial perfusion carries a higher incidence of stroke and other vascular complications (8,13,14) it is frequently used because it is believed that benefits far outweigh risks.
Venous cannulation is performed through the right atrial appendage or through the internal jugular and femoral veins for bicaval access. After institution of CardioPulmonary Bypass (CPB) the aortic cross clamp is applied to the ascending aorta, endoluminally in mitral & tricuspid valve surgery, using the endoballoon (Edwards Lifesciences, Irvine, CA, USA) or externally in aortic valve surgery (11).
Transesophageal echocardiography plays an important role in MIHVS in assessment and repair of valve pathology, canulae placement and de-airing of the heart following the valve procedure.
I - Aortic valve : J-Sternotomy, right anterior thoracotomy or right parasternal incision
Various approaches are adopted, usually as per surgeon’s preference:
1 - J-Sternotomy
The Skin incision is mid-sternum extending from 5 to 7 cms. (Fig -1) Sternotomy is then performed extending from the upper end to the 3rd or 4th inter costal space (ICS) with a J slit to the right half of the sternum (6).
Fig-1, Ministernotomy incision: the incision starts 2 cm cephalad of the angle of Louis.
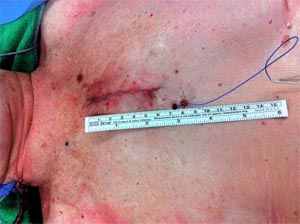
If the sternum is split on both sides at this level it is termed reverse T sternotomy, this may give a better exposure of the aortic root in some patients. With this approach, the internal mammary artery (IMA) is not divided and antegrade arterial and venous cannulation of the right atrial appendage is usually adopted. (Fig- 2)
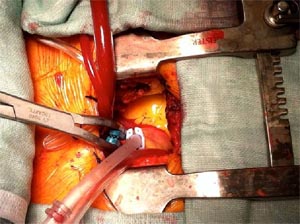
Additional jugular venous access of the superior vena cava (SVC) or bicaval cannulation with femoral and jugular venous access is performed for better venous drainage, depending on surgeon's preference & practice. (Fig-3)
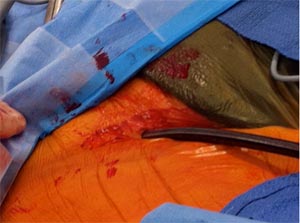
The aortic cross clamp is applied on the ascending aorta. The aorta is then opened, native valve excised and a new prosthesis is implanted.
2 - Right anterior thoracotomy
This constitutes an alternative approach. The incision is made along the 2nd right or 3rd ICS guided by a preoperative CT scan assessment. The incision extends laterally for 5 to 8 cms from the parasternal border (14). The pericardium is entered and antegrade aortic and femoral venous cannulation is usually adopted. This approach typically increases the working distance to the aortic valve, which may create an additional surgical challenge. Advocates of this technique claim reduced postoperative pain. Therefore, this approach has been deemed by some to be less invasive than a J or T sternotomy but given the increased working distance, its application may be optimised by using sutureless valves.
3 - Right parasternal incision
This approach has currently mostly been abandoned. The incision extends from the lower end of 2nd ICS to the superior end of 5th ICS (16). The right IMA is ligated and 3rd & 4th costal cartilages are excised to enter the thoracic cavity. Retrograde arterial and femoral venous cannulation with centrifugal pump to assist venous drainage has been used in this approach. Surgical exposure is adequate to even perform aortic root procedures but disruption of costal cartilages and IMA ligation may cause increased incidence of chest wall instability and related complications (17).
II - Mitral valve: port access, hemi-sternotomy, right parasternal incision or robotic repair
1 - Port access
A 5 to 7 cm incision is made along the infra mammary crease and the thorax is entered through the 4th/5th ICS. Rib spreading is avoided using a soft tissue retractor. (Fig –4)
Fig -4, Minithoractomy- The 5cm incision is held open using a soft tissue retractor. Two other ports for camera and pulmonary venous vent are visible. Carbon dioxide is insufflated into the surgical field through a side arm.
Three ports are generally used and surgery is performed with the aid of a video thoracoscope. (13,15,21). Retrograde aortic cannulation and bicaval cannulation through jugular and femoral venous access is performed. (Fig – 5)
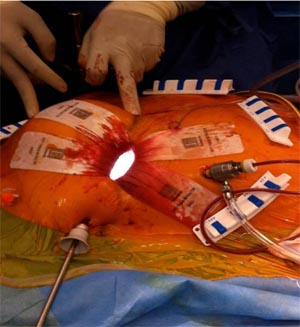
Fig -5, Retrograde arterial cannulation with endo balloon inserted through the side arm of the aorterial canula is used with port access. Femoral vein cannulation is seen medial to the arterial cannula.
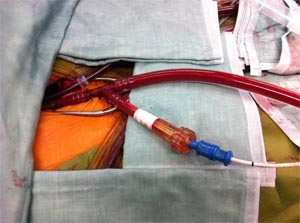
The aortic cross clamp is placed directly on the ascending aorta using a Chitwood clamp (Scanlan International Inc., St. Paul, MN) (11) or an endoballoon clamp. (9,13). Following cardioplegia instillation and cardiac arrest the left atrium is opened and the valve exposed by means of a left atrial retractor. Mitral valve repair is then guided by high definition two-dimension video image assistance. (Fig –4) Port access is now a standardised approach in specialist centres (4,12,13,17). (Fig-6)
Other surgical procedures can also be performed through this approach, these include:
- Ablation for atrial fibrillation (see previous e-journal article here)
- Closure of Atrial Septal Defect (ASD)
- Excision of left atrial tumours such as myxoma
- Interventricular septal wall resection in hypertrophic obstructive cardiomyopathy (HOCM) (22)
Fig -6, Post operative incision and drainage ports, immediately after port access mitral valve surgery.

2 - Hemi-sternotomy
Upper or lower Hemi-sternotomy is also performed to access the mitral valve. The consensus is to minimise sternal splitting. Antegrade aortic cannulation may be possible in upper hemi-sternotomy. Venous access is usually through bicaval peripheral venous access. (19)
3 - Right parasternal incison
As described above, is less commonly used - in cases to approach the mitral valve. Access to the mitral valve is trans septal, cutting through the right atrium and the inter atrial septum. Other approaches described above access through the valve directly by cutting through the roof of left atrium.
4 - Robotic mitral valve repair
This procedure is performed using the da Vinci telemanipulation system (Intuitive Surgical, Mountain View, Calif) through multiple ports. Da Vinci system provides a high-resolution, 3-dimensional video image, allowing remote, tremor-free, and scaled control of endoscopic surgical instruments. (23,24).
The surgical steps are similar to Port access surgery and are adopted to gain exposure to the mitral valve. A Right minithoracotomy of 3 to 5 cms is performed through which the 3-dimensional videoscope is inserted (Fig-7).
Fig-7, Camera port and working ports are shown in this image.
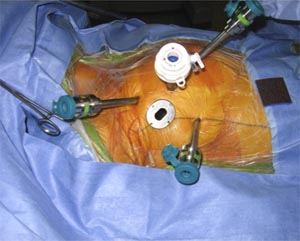
Other instruments are inserted through additional trocars between second to sixth intercostal spaces in the right thoracic cavity. Mitral valve repair is then performed remotely from the surgical console (fig-8). The Selection of an appropriate ring size is based on the preoperative TEE measurement of the free length of the anterior leaflet (longitudinal view) and by intraoperative endoscopic measurements using custom-made sizers.
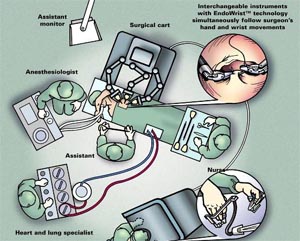
Fig-8, Schematic diagram describing the lay out of Operating Room during Roboric Mitral Valve Surgery. Robotic arms are placed to the right of the patient.
A major disadvantage of the robotic system is the cost involved. Other drawbacks of the robotic method are the loss of tactile sensation and the necessity of an additional table-side surgeon. With the development of adjunct technologies however, the technique of computer-enhanced endoscopic cardiac surgery may potentially become more prevalent (21,23).
III - Advantages and pitfalls
The advantages of minimally invasive heart valve surgery repair are:
- Reduced postoperative pain and early mobility (4,8)
- Reduced blood loss and shorter ventilation time leading to shorter ITU and hospital stay. This in return has been implicated in reducing hospital costs, leading to savings. This however is still debated (4,7,8).
- Less risk of wound infection
- Earlier recovery and return to normal activity (4,24)
- Cosmetically acceptable and patient attractive (8,15)
- Reduces trauma and difficult dissection in redo procedures. This is more appreciated in port access mitral valve procedures through right mini thoracotomy (13).
- Better visualisation using thoracoscope / robot and high definition screen. Enhanced visualisation helps in better insight and training of surgeons.
The pitfalls of MIHVS are:
- Patient selection: may prove more challenging in extreme oversized patients, in patients with Pectus Excavatum (heart may be displaced further into the left thorax) and in patients with previous thoracic surgery. Adhesions following previous thoracic surgery need to be released to create adequate exposure of the surgical field. (For a look at limits to valve surgery by same author, look here).
- Clinical expertise: requires a different skill set, integrating video images into visual feedback and hand eye co-ordination. Using longer instruments and loss of tactile sensation in Robotic enhanced surgery needs practise. All the above implicate a longer learning curve to familiarise with the techniques.
- Vascular complications: may occur following cannulation of femoral vessels. Careful preoperative assessment of iliac artery, femoral artery and aorta undertaken by additional imaging (angiogram/MRI/CT scan) techniques has significantly reduced this type of complication. It is still debated whether retrograde arterial perfusion increases the peri-operative risk of stroke (9,10).
- Injury: The lateral pericardial incision for mitral valve surgery should avoid phrenic nerve traction injury.
IV - Conclusions
The advent of sutureless aortic valves should make minimally invasive aortic valve replacements easier and quicker through less invasive approaches with reduced risk of stroke. The clinical experience with sutureless valves is increasing worldwide. In contrast to transcatheter aortic valve replacement this approach entails removal of all pathological leaflet and annular aortic valve calcification, thus facilitating the implantation of a larger expandable prosthesis. Sutureless bovine valve are constructed on a self-expandable stent, which can be inserted and released to fit in the aortic annulus after debridement of the aortic annulus (26,27,28). For a closer look at sutureless valves, look here.


 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.