Background
Cardiac pacing is the only effective treatment for patients with sick sinus syndrome and atrioventricular conduction disorders (heart block) and its indications have expanded since the development of implantable pacemaker technology.
The right ventricular (RV) apex has been the standard pacing site, for the 600,000+ pacemakers placed in the world each year. However, the deleterious effects of RV apical (RVA) pacing have increasingly been documented in the pacing literature in the last two decades and are thought to be related to the abnormal electrical and mechanical activation pattern of the ventricles (or ventricular dyssynchrony) caused by RV apical pacing.
Deleterious effects include dyssynchronous RV-LV activation, worsening heart failure, adverse ultra-structural myocardial mechanics, deteriorated left ventricular (LV) remodeling and metabolism, worsened LV filling pressures, atrial fibrillation, increased mitral regurgitation, as well as impaired mortality and morbidity. Moreover, chronic RV apical pacing carries risks of short and long term complications including pain, tamponade from perforation, clinically significant far-field over-sensing or stimulation of extra-cardiac structures e.g. phrenic or intercostal stimulation.
We present right ventricular septal pacing as a valid alternative which most mimics what exists in normal physiology. Mond and Vlay, pioneers in the field of septal pacing, have described the new procedure as well as do reviews by Da Costa et al and Shimony et al (1,2,3,4). Lead stability in the hands of sufficiently experienced implanters is excellent, and there are no unforeseen adverse side effect signals for RV septal pacing in patients with normal LV function (5,6,7). The data for non-apical and in particular RV septal pacing, demonstrates improved LV function, prevention of adverse LV remodeling, and prevention of heart failure and contrasts with the evidence of deleterious effects of chronic RV apical pacing in patients with normal and especially reduced LV function (8,9,10,11,12). Read here how septal pacing can be achieved efficiently, accurately, and with long-term success.
I - Pacing site
1 - Right ventricular outflow tract
The anatomical right ventricular outflow tract (RVOT) is defined as the area between the pulmonic valve superiorly, the upper roof of the tricuspid valve apparatus inferiorly, the septum posteriorly and medially, the free RV wall anteriorly and in-between the septum and free wall a small tract of anterior RV wall containing the left anterior descending coronary artery. (Figure 1A)
The proposed target zone for pacing purposes is the septo-parietal trabeculations in the inferior portion of the septal RVOT, or the septal (not free wall) zone bounded by the supraventricular crest, and septo-marginal trabeculation (13,14).(Fig. 1B) 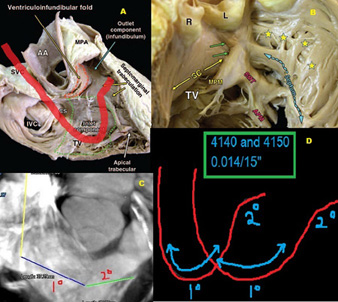
Fig. 1
1A Cadaveric RV RAO dissection showing both inlet and outlet (infundibulum) and septomarginal trabeculations. The proposed course of the lead with the Mond stylet in place has been shown in red. SVC and IVC=superior and inferior vena cava; AA=ascending aorta; MPA=Main pulmonary artery; CS=coronary sinus.
1B Endocardial view of the TVOT and high septum showing the septoparietal trabeculations (stars) arising from the septomarginal trabeculation (SMT). Underlying crisscross architecture of myocardial strands allows good lead anchoring. TV=tricuspid valve; MPM and APM= medial and anterior papillary muscles; SC=supraventricular crest; SMT=septomarginal trabeculation
1C Reconstructed 64 slice cardiac CT view of the RVOT showing the exact course of the Mond stylet from SVC to RA to septum/SPT.1D Two versions of the Mond stylet available with demonstrated primary and secondary curves.
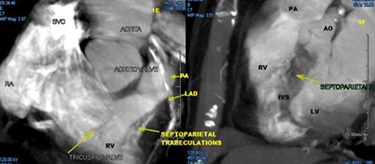
Fig. 1E,1F
Reconstructed 64 slice views of the right ventricular outflow tract showing the exact area of the septomarginal and septoparietal trabeculations which are the proposed target areas for septal non-apical RV pacing, demonstrated by angiographic contrast injection (also shown in Fig.4B)
Indeed, the zone at or below the level of the moderator band is too close to the apex and the high RVOT septum in the infundibulum/conus arteriosus is smooth walled and yields poor attachment of leads and high pacing thresholds.
2 - Rationale and physiology
Using electrocardiographic imaging (ECGI) in which a 224 ECG map of the entire torso surface is anatomically coupled to a high resolution CT scan image of the heart, Ramanathan et al mapped the earliest cardiac electrical activation sequences, finding a general cardiac electrical activation sequence common to humans which involves earliest epicardial activation in the right paraseptal region and multiple breakthroughs aligned parallel to the course of the left anterior descending coronary artery (15). (Figure 2)
The septo-parietal target zone for non-apical RV septal pacing is proposed on this anatomical-physiological basis.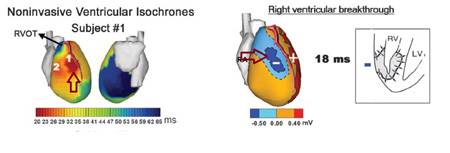

Fig. 2
Left Inset: Noninvasive ventricular isochrones (activation sequences) using electrocardiographic imaging to image epicardial potentials-Location of earliest activation site in the RV right paraseptal zone within 25msec of QRS onset—elliptical areas of activation can be observed in many subjects parallel to the LAD coronary artery course.
Right Inset: RV breakthrough shown as an intense potential negative minimum at the corresponding right paraseptal zone illustrated adjacent.
3 - Modified Mond lead
Delivery of pacing leads to the appropriate location in the RVOT from the subclavian approach (most common approach) via the SVC requires a stylet shaped with primary and secondary curves (instead of the regular straight stylet). The primary curve is required to traverse the tricuspid valve towards the RVOT away from the apex, and a secondary posteriorly curve then redirects the lead towards the septum away from the anterior free wall (Fig. 1C, 1D).
Fig. 3
Mond Stylet St Jude™ illustrating the 3-dimensional posterior angulation of the secondary curve compared to the primary curve.
These features were identified by Vlay and modified by Mond who has validated the use of this stylet in a large case series (16). While this stylet shape can be approximated manually, the commercial availability of the St. Jude Medical® , Inc., St. Paul, MN, USA Mond stylet has resulted in greater ease, accuracy and reliability in septal lead delivery. (Figure 3) The primary curve is simple and serves to deliver the lead to the septal /outflow vicinity, while the posterior directionality of the secondary curve steers the lead away from the free wall towards the target zone septo-marginal trabeculations that offer a stable implantation site. (Figure 1C, 1D) Using a 3D shape similar to the Mond™ style, and a right anterior obliqe (RAO) fluoroscopic grid target site, Burri et al, using echo for confirmation of location, showed a 97% success rate for mid-septal positioning could be achieved. This compared with a 45% success rate using a 2D shape (Vlay-S type with no posterior bend) and no RAO grid target site overlay (17). In a contemporary 2013 series of 51 patients, Osmancik et al utilised a manually fashioned 3D Mond-type stylet to deliver pacing leads to a mid-septal fluoroscopic target, and then verified the lead location using 256 slice cardiac CT (18). They found 59% were in the anterior wall and only 41% in the true target mid-septal location. Clearly, if we are to improve reproducibility of positioning, either standardisation of the stylet shape (rather than manual ad hoc shaping), and/or alternative imaging guidance to the target site is required to increase reproducibility and success rates.
4 - Steps to the technique, using angiography
The technique we present ensures highly accurate reproducible positioning, and relies on anatomical identification of the target zone using angiography, with only confirmatory reliance on less accurate positional indicators such as the fluoroscopic views or 12 lead ECG vectors.
1) Set-up
Separate dual sheath venous access (presumed to be subclavian for this discussion) is established. Once venous access is established, a simple curved soft stylet is placed in the lead, and the ventricular lead delivered using one of the two sheaths, through the tricuspid valve towards the RVOT and then across the pulmonic valve into the left or right main pulmonary artery. A longer length lead with sufficient slack allows prevention of lead prolapse during stylet exchanges as described below. This is particularly important in cases of tortuous venous anato
my, large RV size and large body size. (Figure 4A)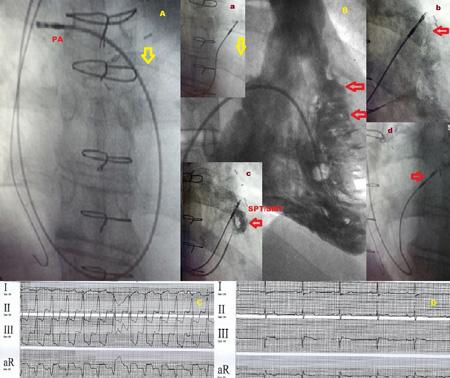
4A Initial position of the RV lead in the right main PA achieved buy using a simple shallow curved stylet to advance from RA to RVOT and then to PA.
4B Comparison RV ventriculogram showing the angiographic location (open arrows) of the septoparietal trabeculations (refer Figs. 1A-1C). Inset a RVOT septal lead being dragged inferiorly with Mond stylet in place from PA to septum. Inset b Lead screwed into place into SPT zone identified during pull back contrast injection. Inset c confirmation of lead location by a small bolus of contrast injection clearly delineating the SMP/SPT zone described above. Inset d Steep (left anterior technique) LAO view showing lead in position against high septal location.
4C,4D Paced and unpaced ECG leads showing the isoelectric/slight positive lead I vector and inferior axis of the paced ECG.
2) Stylet exchange
The simple curve is exchanged for the dual curve Mond stylet (St. Jude type). A soft stylet and careful advancement techniques are suggested until the Mond stylet is placed fully in the lead which still rests in the pulmonary artery (PA).
3) Mapping the target zone
A balloon flotation end-hole injection catheter (e.g. coronary sinus injection catheter) is advanced with balloon inflated into the main PA across the pulmonic valve. A 10 cc luer lock syringe with 100% contrast is attached to the injection port. The catheter is then pulled down to just below the pulmonary valve using a small puff contrast to verify initial position. A pull back hand injection angiogram is then performed by firmly injecting and simultaneously dragging the balloon catheter (with balloon inflated) from RVOT down into the RV cavity across the septum. (Figure 4B) A road map is created of the target zone septo-parietal trabeculations and placed on the monitoring reference screen. We perform this pull back using the AP view, but it is best mapped by using an RAO view also. In fact, a “rolling” cine sequence from RAO to LAO with sustained injection yields the best three dimensional appreciation of the location of this area. With recurrent practice, only a single confirmatory AP view may suffice if a Mond type stylet and the above pull back technique is utilised. Alternatively, providing neither the image intensifier nor the table are moved, the TZ can be marked on the monitor screen and used as a guide for step 4 below. A better approach is to access with two sheaths initially allowing one to be used for balloon catheter introduction, and the other for ventricular lead insertion. This also allows for verification of lead position or guidance of lead repositioning if required by repeat puff contrast injection as shown in (Figure 4 inset e).
4) Lead delivery
The Mond stylet preloaded lead is now dragged back from the main PA and is automatically directed towards the septum away from the free wall. With minimal manipulation the target zone is selected by the lead and screwed into place (active fixation leads are preferred, though passive fixation can be used also). (Figs.4B insets a and b) If the target zone is not easily selected, the lead is replaced in the main PA and the Mond stylet removed for reshaping the primary or secondary curves prior to re-introduction and re-fixation using the same drag down procedure described. This usually can truly be accomplished swiftly in under 5 minutes which negates the idea of a complex time consuming procedure and is therefore clinically useful.
5 - Confirmation
Finally the regular RAO and LAO 40 degree views and the 12 lead ECG vectors can be utilised to confirm the positioning accuracy. The main points for fluoroscopic evaluation are the septal direction (right ward and posterior pointing directionality towards the spine) of the v-lead away from the anterior free wall in the LAO view (Figures 4 inset d, 5). According to published literature, ECG vector criteria include (19,20) 1) A vertical frontal axis with deep S in lead I (sometimes equivocal /inferior) and a clock wise rotation of the horizontal axis with tall R waves in leads V4-V6. 2) In cases of accidental free wall implantation, smaller R waves are seen in V5-V6 and the upright R wave is seen in lead I. a notched R wave in lead III is also frequently seen in cases of free wall pacing (21). (Figure 5) Allowing for variations in heart rotation and vertical position in the thoracic cavity, none of these ECG criteria may be fail-safe. Burri et al using electro-anatomic mapping of the RV to confirm pacing lead location, showed in 31 patients that no single ECG criterion was reliable in distinguishing mid-septal from anterior free wall locations, and that in fact a negative Q RS or q-wave was in fact more frequent in anterior rather than mid-septal location suspected by ECG vector analysis, fluoroscopic imaging in a steep LAO or left lateral view helps to confirm this (Figs 4D, 5).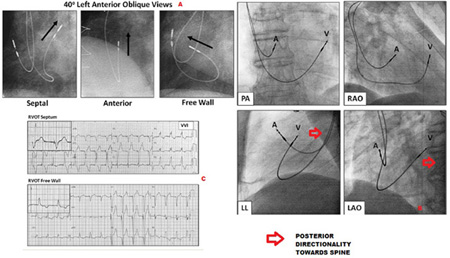
Fig. 5
5A 40º left anterior oblique fluoroscopic views of RV lead showing the importance of the lead tip pointing towards the vertebral column (arrow) as opposed to anteriorly which redirects the lead towards the free wall and more anterior location.
5B PA RAO 40º and LAO 40º views help locate the ventricular lead on the RVOT septum. LO oblique and lateral views show the posterior and septal directionality of the lead insertion.
5CForced VVI pacing showing the ECG criteria differentiating RVOT free wall from RVOT septal locations based on the a deep S in lead I, and tall R waves from V4-V6. This contrasts with the tall R of lead I (more leftward frontal plane), and clockwise rotation of the horizontal axis resulting in smaller R waves in V5 and V6 only. The specificity and accuracy of these ECG criteria is discussed in the text.
6 - Alternate lead delivery: catheter based techniques
An alternative delivery technique using the Select Secure®, Medtronic Inc. Minneapolis, MN USA) lumen less actively fixed screw in 4.1 F lead and the Select-Site steerable guide or the C315 type fixed shape catheter delivery system is available. (Figure 6) 
Fig. 6
Select Secure®, Medtronic Inc. Minneapolis, MN USA) lumen less actively fixed screw in 4.1 F lead and the Select-Site steerable guide or the C315 type fixed shape catheter delivery system.
There is a significant learning curve and in our experience this alternate lead delivery has been more cumbersome than the stylet based approach which is faster, more convenient and reproducible. The fixed shape peel away coronary sinus delivery catheter can be used for lumen less lead delivery. However, not all these fixed or deflectable type delivery catheters possess the required posterior tip angulation to avoid the free wall (which is crucial to avoid pacing induced dyssynchrony) and their reproducibility in achieving the target zone for RVOT/mid-septal pacing, is not established. In the largest published series of 138 ventricular implants, using the Select Site ®, Medtronic Inc. system, Zanon et al found a 98% success rate in implanting RVOT leads with a mean fluoroscopic time of 19±15 minutes, suffering one perforation, and two acute lead dislodgments (22). As judged by LAO and RAO views as well as ECG vectors, 76% achieved a high RVOT, 6% a free wall RVOT and 18% a low septal position.
Indeed, the septal stylet delivery technique has been extensively tested and validated, and is usually achieved in experienced hands with fluoroscopic times under 5 minutes. Vlay described a 9-year experience in PACE 2006, of 460 patients with RVOT-septal pacing with stylet driven delivery, with a 92% success rate and only one dislodgement out of 460 patients and no significant differences in thresholds for sensing/pacing or lead impedance between apical and RVOT pacing subgroup comparisons. Likewise Mond and Vlay cite a greater than 1,200 case experience RV stylet driven delivery of active fixation leads to the RVOT and mid-septum, with an over 90% success rate, specifically 89% for the mid RV septum and 97% for the RVOT2. A 1-2% failure rate is seen with immediate postoperative dislodgements (decreases with experience), and with cases of gross RV enlargement, or torrential tricuspid regurgitation. In Mond’s 2009 (Pacing Clin Electrophysiol) reported RVOT series at 1 year the average stimulation threshold was 1.5 Volts with 94% of the leads having a <1 Volt stimulation threshold (23). Moreover, unlike RV apex pacing complications such as high threshold exit block, diaphragmatic pacing and pericardial pain or tamponade are all rare to non-existent.
7 - Septal lead defibrillator positioning
It should be further noted that the current randomised literature for dual chamber implantable cardioverter defibrillator (ICD) shows no significant differences in capture, delivery success or defibrillation thresholds between septal or RVOT defibrillator lead placement versus conventional apical insertion (24). In the largest reported series, 185 patients in the EFFORT registry, using a mixture of devices and leads from Medtronic™, St, Jude™, Boston Scientific™, Biotronik™, and Sorin™, Mascioli et al show similar efficacy and safety between RVOT and RVA ICD placement of defibrillator leads, similar pace/sense thresholds, and similar efficacy in a 14J shock restoration of sinus rhythm from VF induction (25). RVOT defibrillator insertion with 7F compatible dual coil leads is easily achieved in the high mid septal / RVOT locations using the technique we describe.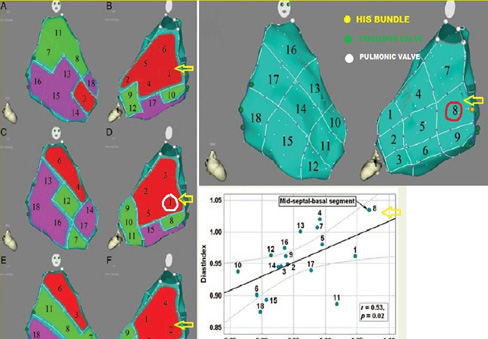
Fig. 7
Hemodynamic effects of pacing from differing RV pacing location
Left panel shows free wall (RAO) and septal (posterior-anterior views) on a CARTO electroanatomical map. His bundle (yellow circle) , TV (green circles) and PV (white circles) are shown. Arrow indicates mid-septal basal segment.
Right panel shows the best RV pacing site based on indices of systolic and diastolic performance is the mid-septal-basal segment indicated by arrow.
II - Evidence favoring non-apical RVOT-septal pacing
1 - Hemodynamics
In a 2003 pooled analysis of 217 patients from 9 studies, de Cock et al reported a significantly better hemodynamic effect of RVOT vs. RVA pacing (odds ratio 0.34 CI 0.15-0.53) but heterogeneity in pacing effects, sample sizes, chronicity and assessment methodology were inherent (26). Using invasive measurements of LV and RV systolic and diastolic function, as well as 3D electro-anatomic mapping with the CARTO system, Biosense Webster™, Vančura et al showed using a comprehensive mapping protocol in eight patients, that comparing single site endocardial pacing (lateral LV region vs. differing RV sites within the 3D RV map) that the best indices of LV systolic/diastolic/pulse pressure function were obtained by pacing the RV at superior-mid-septal segment of the RV (27). (Figure 8) 2)Interventricular mechanical synchrony and ejection fraction 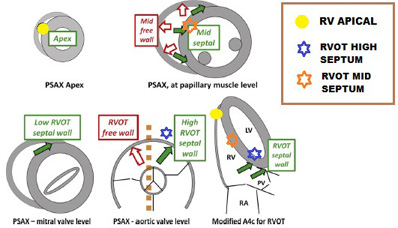
Fig. 8
Echocardiographic criteria to determine lead position. Dashed lines represent the plane of the interventricular septum. Open and solid arrows indicate the relative positions of the free wall and septum in cross sectional views. Differentiation between high low and mid septal wall locations by differing views is illustrated.
2 - Interventricular mechanical synchrony and ejection fraction
Following AV node ablation, Victor et al studied 28 patients and showed narrower QRS and at 3 months, better preserved LVEF than chronic RV apical pacing (28). Using RV pacing from the mid-septum and RVOT, Alhous et al have shown improved LVEF compared with RVA pacing and reduced pacing induced dyssynchrony indices compared with the RVA (8). In a longer 12 month study of 60 patients, Wang et al also showed superiority of RVOT vs. RVA pacing for LV regional performance, LV global electromechanical delay, and inter-ventricular mechanical delay (29). Interestingly, no difference in EF was seen suggesting that chronic evaluations of this parameter may need to extend to greater than 1-2 years. In a meta-analysis of 14 RCT’s/754 patients, Shimony et al found a better LVEF in non-apical vs. apical RV pacing particularly in patients followed for more than 12 months, and with LVEF≤ 40-45%4. Similarly,Tse et al demonstrated a progressive deterioration between 6 and 18 months of follow up in incidence of myocardial perfusion defects, regional wall motion abnormalities, and LVEF for RVA vs. RVOT pacing (30)
3 - Clinical endpoint
Riahi et al using the DANPACE study cohort of 1,415 patients with sick sinus syndrome randomised to AAIR vs. DDR pacing, investigated the occurrence of heart failure in >5 years follow up (31). These authors were unable to demonstrate any correlation between the incidence of heart failure and % V-pacing or lead location (apical vs. non-apical). However, heart failure was clinically determined, lead location was non-randomised, and neither serial echocardiography nor BNP corroboration of heart failure status was available. Moreover a single frontal plane, fluoroscopic view was used to determine lead positioning with no other imaging confirmation of lead position. Thus, while the DAVID and MOST trial have clearly demonstrated increased risk of heart failure hospitalisation and/or death from increased burdens of RV apical pacing, the opposite demonstration of benefit from non-apical (specifically septal or RVOT) pacing has been confounded by methodological deficiencies and lack of sustained follow up. Despite much anticipation, after almost 5 years and after completion of recruitment, we still await the results of two alternative site multicenter randomised pacing trials (32): (1) PROTECT-PACE The Protection of Left Ventricular Function During Right Ventricular Pacing (2007 initiated) comparing RV apical vs. high septal pacing for primary EF and secondary heart failure/arrhythmias endpoints (33). (2) RASP Right Apical Versus Septal Pacing Trial (2005 initiated) comparing RV inflow tract septal vs. apical or EF primary and mortality, arrhythmia and heart failure 6 minute walk QoL endpoints (34). The OPTIMIZE-RV trail of mid-septal vs. RV apical pacing initiated 2007 has been terminated for speculated reasons of lack of recruitment or perhaps methodological issues (35). While the lack of well derived clinical outcomes data renders definitive conclusions impossible difficult, well designed randomised single center study data are now emerging. Using a double blind randomised design and a cohort of 142 patients with advanced atrioventricular block, Molina et al elegantly demonstrate 1 year outcomes data showing superior and significant improvements in LVEF, 6 minute walk distance with septal versus apical RV pacing.
III - Cardiac resynchronisation therapy using ROT lead positions
Only a few studies have examined the role of RV lead position in the efficacy and outcome of cardiac resynchronisation therapy (CRT). In a single blind prospective randomisation of 53 patients to RV septal vs. RV apical lead positioning guided by RV mapping to obtain maximal electrical separation (MES) between the LV and RV leads, Miranda et al elegantly show at 3 months that the RV-LV MES was greatest in septal locations and corresponded to a greater percentage of CRT responders as judged by EF, 6 minute walk test, with no adverse safety issues. The multicenter SEPTAL-CRT study comparing mid-septal RV lead CRT has completed recruitment but not yet reported results for clinical endpoints. In other studies, Kristiansen et al have shown similar reverse remodeling and reversal of LV dyssynchrony at 6 months between RV apex and high posterior septal RV implant as adjudicated by orthogonal view fluoroscopy (36). The confounding effects of possible RV lead displacement and discordant LV lead placement, due to anatomical factors makes interpretation difficult. Rönn et al using a randomised design in 33 patients with classical CRT indications could not show a difference between apex and RVOT RV lead positioning in endpoints of EF, QoL, peak oxygen uptake, BNP, or 6 minute walk (37). The single adverse signal of non-apical RV lead location in CRT comes from a retrospective analysis by Kutyifa et al using the MADIT-CRT database, in which an excess of the endpoint of either VT/VF or VT/VF/death was ascribed to non-apical RV lead location with no difference in the primary endpoints of heart failure or death (38). Clearly more prospective randomised data with appropriate methodology are needed.
IV - Alternative imaging modalities to confirm/guide implantation site
Echocardiography can be used to locate RV pacing sites (Figure 8) and to assess the dyssynchrony associated with pacing from these sites. However, such evaluations are difficult to achieve intra-procedurally with sterility in the operating room or catheterisation laboratory, and are more time consuming and expensive in terms of equipment and personnel also. Using speckle tracking based global longitudinal strain assessment (important predictor of outcome in chronic LV dysfunction), Inoue et al showed that QRS width and both longitudinal and radial strain indices of dyssynchrony were worse in the apical than septal pacing location (39,40). Lastly, while 3D echo and intra-cardiac echo are promising in the visualisation of putative RVOT septal pacing sites, the resolution of 3D imaging is a work in progress, and the time/expense and cumbersomeness of both techniques is prohibitive on a routine basis (41). The resolution of contrast angiographic techniques and cardiac CT for localisation are clearly superior and more facile to achieve.
As well as being intuitive, convenient and fast, the anatomical identification of the septoparietal and septomarginal trabecular zone of the RV septum by contrast angiography offers two major advantages: 1) A high resolution target image for accurately manipulating the RV lead to the desired location 2) Avoids reliance on fluoroscopic views and ECG vectors which while useful as confirmatory techniques, have significant fallibility in identifying accurate septal vs. free wall or even mid vs. high septal positioning. The disadvantage of using a balloon catheter and 10-15cc’s of contrast seems small, in comparison to the advantages offered. A suggestion is for the Mond stylet and RVOT angiographic mapping to be prospectively tested for accurate anatomical positioning using a “gold standard” imaging technique such as cardiac multi-slice computerised tomography (or cardiac MRI for MRI safe devices). Because of the complete three dimensional data set available, reconstruction in any desired view is feasible, (Figs. 1E,1F) scanning time is fast for cardiac CT, the scan is gate-able with a regular rhythm (paced or otherwise), and the radiation dose may be limited by using the smallest scan window between the pulmonic valve superiorly and the RV apex inferiorly. Moreover LV and RV function can be quantitated using CT or MRI. The elegance of such an approach is that for the first time it will allow the clinical endpoints to be exactly analysed according to the anatomical site of lead insertion. In fact, predicting the best physiologic response to non-apical septal or RVOT pacing is predicated on accurately and reproducibly finding the best target zone for pacing. Many of the equivocal studies for long term RV septal or outflow tract (vs. RV apical) pacing have been attributed in significant degree to the improper localisation of septal leads, particularly when the mistaken implantation occurs in the anterior free wall. In this case, the old real estate maxim of “location, location, location” holds never truer.
V- Possible future pacing trial design
What is desperately needed for the pacing community to convince reluctant adopters of RV septal (non-apical) pacing, is a long term multicenter randomised clinical study that uses image.


 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.