Background
Ischaemic heart disease (IHD) - reduced blood supply to the heart usually caused by coronary artery disease - is the leading cause of death and morbidity in America and Europe and is expected to be so by the year 2020 in emerging countries as well (1-3).
Percutaneous coronary intervention, coronary artery bypass grafting and medical therapy have had an outstanding impact to help reduce the burden of IHD, nonetheless, certain patients may continue to have angina symptoms and will experience:
- Insufficient revascularisation: Revascularisation can be impossible to achieve such as in cases of peripheral coronary pathology.
- Ineffective drugs or side effects: Conventional drugs such as b β-blockers, calcium-channel blockers, molsidomine, nicorandil and ivabradine, can be not effective enough or their use limited by side-effects.
- Absence of coronary artery disease: Cardiac ischaemia with angina pectoris can exist in the absence of significant coronary artery disease, possibly due to microcoronary dysfunction (up to 10% of patients of which a majority of women).
Ranolazine, an anti-ischaemic agent with additional electrophysiological properties can help in the above-described situations and is different from conventional agents (4) in that it does not reduce heart rate nor blood pressure.
1 - Known mechanism and approved use
Ranolazine is, like trimetazidine, a piperazine derivative - piperazine itself was introduced in 1953 as an anthelmintic - a drug to expel parasitic worms in the body. Ranolazine was developed in the late 1990s and has been approved as a anti-anginal medicine subsequently.
Unlike trimetazidine, another antianginal drug which is also a piperazine derivative for which the European Medicines Agency (EMEA) has recommended in 2012 to restrict the use of to second-line therapy, Ranolazine has been approved as add-on therapy for the symptomatic treatment of patients with stable angina pectoris who are inadequately controlled or intolerant to first-line antianginal therapies such as beta-blockers and/or calcium antagonists by both the Federal Drug Administration (FDA) in 2006 and by the EMEA in 2008 and as first-line therapy by the FDA in the treatment of chronic angina in 2012.
The main studies carried out with Ranolazine in angina were:
- MARISA (5) (2004; 191 patients) Monotherapy Assessment of Ranolazine In Stable Angina trial was a dose-response study that showed increasing doses of razonaline increased exercise duration by 94, 103 and 116 seconds.
- CARISA (6) (2004; 823 patients) evaluated ranolazine plus anti-anginal treatment against placebo plus anti-anginal treatment. Patients treated with ranolazine had significantly better exercise duration - an ncrease by 115 seconds from baseline versus 91 seconds in placebo group - time to onset of angina, and fewer angina attacks.
- ERICA (7) (2006; 566 patients) evaluated ranolazine plus amlodipine against amlodipine alone. Weekly consumption of short-acting nitrates was significantly lower in patients who received ranolazine plus amlodipine versus amlodipine alone.
- MERLIN-TIMI (8) (2008; 3,162) Metabolic efficiency with ranolazine for less ischemia in Non-ST elevation acute coronary syndromes compared long-term treatment with extended-release ranolazine with placebo, on top of standard therapy, for acute and long-term treatment of patients with non-ST-elevation ACS. Although cardiovascular death, myocardial infarction or severe recurrent ischaemia were not proven better with ronalazine, the individual component of recurrent ischaemia was significantly reduced by ranolazine - demonstrated as safe.
Regarding the mechanisms of ranolazine, reduced diastolic myofilament activation is one mechanism of ranolazine which has been demonstrated - this action is achieved at therapeutic levels which have been determined at 375-750 mg twice a day, through inhibition of the cardiac late Na+ current (INa+) and reduction of the Ca2+ overload. Cross-bridge kinetics of the cardiomyocytes are ameliorated as a result (9-11) and thereby assuming a prodiastolic dysfunction.
Some researchers believe that reduced diastolic myofilament activation's net result is reduced oxygen consumption and reduced wall tension thereby improving microvascular blood flow however its value with regard to its antianginal and anti-ischaemic mechanism remains speculative. Additionally, ranolazine has been found to inhibit the IKr-current (12) which is what might explain its electrophysiological effects.
Ranolazine also prolongs the QTc-interval, but shortens repolarization in type-3 long-QT-syndrome (13). It is not indicated however to treat patients with cardiac arrhythmias or for lowering haemoglobin A1c in diabetic patients (4). It was also confirmed not to promote sudden death in patients with long-QT syndrome (14). Ranolazine is used today in its extended-release formulation only (15).
2 - Off-label use and case report
A recent review and its accompanying editorial analyse the present knowledge on ranolazine in cardiac diseases and put forward that ranolazine is indicated in patients with stable IHD and refractory angina pectoris (4,16) added to a β-blocker and/or calcium channel blocker in patients without a background of long-acting organic nitrates who remain symptomatic despite treatment with these agents.
Furthermore, it is thought that ranolazine should be used as initial antianginal therapy (instead of treatment with a β-blocker, or a calcium-channel blocker, or a nitrate preparation) when an absolute or a relative contraindication to treatment with these agents is present, and if there is a concern regarding low blood pressure or low heart rate.
- IHD patients with diabetes or metabolic syndrome (17-19).
- Atrial fibrillation and other arrhythmias (20-22).
- Symptomatic IHD patients with left ventricular dysfunction (4,9).
Her weight and blood pressure were normal. A laboratory check-up did not reveal any relevant pathology, the thyroid function was normal.
The ECG revealed a negative T-wave in I and aVL (Fig. 1a).
At echocardiography the left ventricle had normal dimensions, was non hypertrophic, had a normal regional contraction, normal systolic function (EF 67%); there were a minor left ventricular relaxation dysfunction and a moderate mitral regurgitation (Fig. 2a), without enlargement of the left atrium and with a normal pulmonary pressure.
Stress-echocardiography did not unmask dyskinesia and the ejection fraction increased by 6%. A coronary angiography and ventriculography were performed: the coronary arteries were normal, the left ventricle had a normal size, non-dyskinetic and the ejection fraction was normal (68%), the mitral regurgitation was mild to moderate.
Therapy with a β-blocker had insignificant effects on cardiac symptoms and at a very low dosage, induced bradycardia (resting HR < 52/min) with dizziness.
Verapamil was ineffective and was discontinued because of severe constipation.
Diltiazem was ineffective and induced a pitting edema of the ankles.
Nitrates and nicorandil were ineffective and were discontinued because of severe headache and migraine.
Ivabradine reduced sinus rate by 5 beats/min) but had no effect on cardiac symptoms.
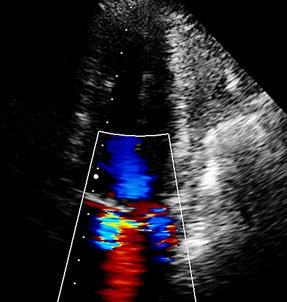
A therapy with ranolazine was started and the dose was increased without side-effects to 850 mg; within 3 months the patient was symptom-free. The ECG showed the T-waves had become normal (Fig. 2a) and echocardiography revealed a normal left ventricle (unchanged) but the severity of mitral regurgitation had clearly decreased (Fig. 2b).
The case presented shows a real aspect of cardiology life: Approximately 10% of patients (>90% female and over 55-year-old) have typical angina pectoris and pathologic findings in the ECG and echocardiogram.
However, when they undergo coronaro-ventriculography one finds normal epicardial arteries.
Microcoronary dysfunction cannot be detected under diagnostic procedures. The pathology is called “cardiac X syndrome” or, as I personally prefer, cardiac ischaemia with normal coronary arteries (23).
These patients have ischaemic symptoms and signs and do not respond (in >80%) to conventional drugs. Off-label use of ranolazine is particularly well-suited for these patients.
Fig. 1a: Baseline ECG: negative T-wave in I and aVL.
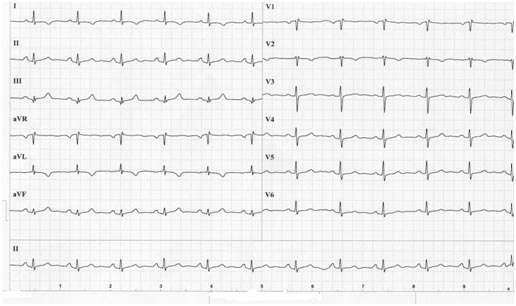
Fig 1b: Baseline echocardiogram: jet of mitral regurgitation into the left atrium.
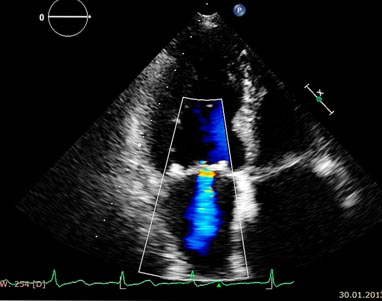
Figure 2a: The T negativity is disappeared.
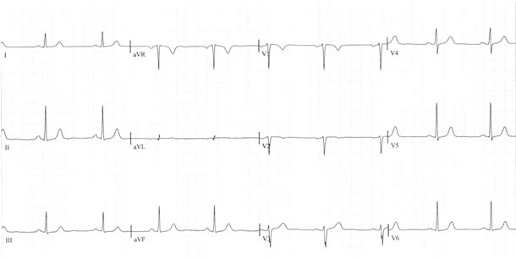
Figure 2b: Echocardiogram: The jet of mitral regurgitation has decreased.
3 - Interactions, safety issues and cost
Many pharmacologic interactions with ranolazine have been reviewed (4) however the list of interactions is neither complete nor final (16).- Colchicine: An important interaction exists between ranolazine and colchicine. Both drugs inhibit the P-glycoprotein (P-gp) and their co-administration may significantly increase the serum concentrations of colchicine by enhanced absorption as well as reduced excretion of colchicine due to inhibition of P-gp efflux transporter in the intestine, renal proximal tubule, and liver.
- Digoxin: One should be cautious when using ranolazine with digoxin (16) because ranolazine may increase digoxin’s serum concentration.
One must note that compared to conventional antianginal agents, ranolazine is not cheap.
4 - Future Studies
There are plenty of studies regarding ranolazine in very diverse areas of cardiology however the most numerous are those regarding ischaemic heart disease, angina and myocardial disease together with those regarding rhythmology of which, the HARMONY trial - A Study to Evaluate the Effect of Ranolazine and Dronedarone When Given Alone and in Combination in Patients With Paroxysmal Atrial Fibrillation and the RAFFAELLO trial - Ranolazine in Atrial Fibrillation Following An ELectricaL CardiOversion (headed by J.Camm) which are both due for preliminary results this year.
Safety of Amiodarone and Ranolazine Together in Patients With Angina SARA will test the difference in arrhythmia burden.
Ranexa versus placebo in patients with ischaemic (due to blockages) cardiomyopathy treated with optimal conventional medical therapy and/or percutaneous revascularisation is recruiting patients and Ranolazine in Ischaemic Cardiomyopathy Patients With Persistent Chest Pain or Dyspnoea Despite Conventional Therapy: A Cross-Over Study is also recruiting patients.
Conclusions
Cardiac ischaemia with angina pectoris can exist in the absence of significant coronary artery disease, possibly due to microcoronary dysfunction (up to 10% of patients of which a majority of women). Conventional anti-ischaemic drugs may be disappointing in the therapy of this condition and ranolazine may be a particularly interesting approach in these patients.
Furthurmore, ranolazine's indications could be extended to:- Angina pectoris and myocardial ischaemia without coronary artery disease (cardiac X syndrome)
- Symptomatic patients with IHD and diabetes mellitus and/or some arrhythmias
- Symptomatic patients with IHD and diastolic dysfunction


 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.