Background
Advances in noninvasive exploration of the unborn child offer better reporting of congenital heart disease in utero, and have allowed for more children with congenital cardiac malformations to receive the care they needed and go on to grow to have children themselves. All in all, congenital heart disease accounts for 80% of all cardiovascular disease in Western Europe and North American pregnancies, as opposed to only 10% in other countries (1). Increasingly, modern child care starts in intrauterine life. As a result, prognosis is improved from earlier diagnosis. For example, in first trimester pregnancy high time and spatial resolution in B mode (0,1-0,01 mm), color Doppler, power color Doppler, and spectral Doppler with gate as little as 0,5 mm give us the chance to obtain detailed assessment starting at 11 weeks of gestational age as part of a routine screening practice and starting at 13-14 weeks for specialised echocardiography evaluation (4). Here we point out risk of cardiovascular congenital anomalies and achievements in intrauterine fetal evaluation.
Risk
All pregnancies hold a risk of congenital anomalies. With brain anomalies, abnormalities of the heart are the most frequent, however incidence and risk vary according to parental history and pathology. Risk varies:
- In low risk pregnancies (fewer than 3 cardiovascular risk factors) the incidence of cardiovascular fetal disease is around 1% of all live births (2).
- In high risk pregnancies (patients with previous recurrent venous thrombo-embolism (VTE), or estrogen-related or unprovoked VTE, or single previous VTE + thrombophilia or family history), there is parental disease or affected siblings and risk of varies from 3% to 50% (1), depending on 1) genetic or chromosomal involvement of affected parent or sibling, 2) pattern of inheritance as well as 3) other maternal diseases or conditions.
- Maternal conditions: diabetes, phenylketonuria (a metabolic disorder), autoimmune disease (anti-Ro / SSA, anti-LA / SSB) infection with parvovirus B19, coxackie, rubella, teratogen exposure - retinoids, phenytoin, carbamazepine, lithium, valproic acid etc., exposure to non steroidal anti-inflammatory drugs - ibuprofen, salicylic acid, indomethacin- and in-vitro fertilisation (2, 3).
In all live births, the number of pregnancies at high risk is
small that is why the actual burden of overall congenital cardiovascular anomalies actually lies in low risk pregnancies because the number of affected children is greater: (1% of 100,000 low risk live births is 1000 cardiovascular anomalies while 5% of 10,000 high risk live births is 500).
I - Genetic testing
Prenatal genetic testing is justified when outcome can change such as through 1) intervention, 2) termination of pregnancy (TOP) or 3) setting up individualised specialised care - including in utero treatment.
However, justification for prenatal genetic testing is a matter of debate because:
- Risk of miscarriage: the fetal/placental unit must be invaded which implies a risk of miscarriage of about 0,5-1% both for chorionic villus sampling (placental biopsy at 11-14 weeks) and for amniocentesis (amniotic fluid collection at 16+weeks).
- Outcome: Outcome does not change as a result of testing itself either for karyotype or for specific genetic testing (trough FISH, QfPCR or CGH array) but puts forward the choice for TOP in some cases (aneuploidies, DiGeorge syndrome etc. with documented structural anomaly).
- Correlations with phenotype: epigenetics, which are heritable changes in gene expression or phenotype caused by changes in the chromosomes other than changes in the underlying DNA sequence, and other factors cause the final phenotype to vary widely and disease to impact affected children in various forms. Thus, genetic test results do not strictly correlate with phenotype, therefore deciding to terminate pregnancy is questionable.
- Change in care: Positive genetic tests do not change pregnancy care because the pregnancy is already high risk; high risk is what initially prompted the genetic testing in the first place.
In postnatal care, for a patient with detected anomaly or positive genetic testing, preventive care can be provided in terms of lifestyle and healthcare benefits that will help for a better prognosis and to provide a milder extent of the disease and timely intervention.
For a look at genetic testing in cardiomyopathies, see here a previous edition of e-journal on the topic.
II - Ultrasound
A- Echography
Since congenital cardiovascular anomaly is a phenotypic concept, the tools that can assess structure (morphology) and function are of great use. In utero, one of the best and harmless tools is ultrasound examination of the fetus and its annexes. Indeed, offers improved:
- Timing: With high performance equipment, skilled and experienced examiners, echocardiography can be performed with remarkable results as early as 12-13 weeks of pregnancy (4).
- Detail: High time and spatial resolution in B mode (0,1-0,01 mm), color Doppler, power color Doppler, spectral Doppler with gate as little as 0,5 mm give a chance to have detailed assessment starting at 11 weeks of gestational age still within first trimester diagnosis that allows for timely intervention.
- 3D: From second trimester of pregnancy as it is limited in time and spatial resolution, three dimensional technology offers improved resolution and spatial relations, thus better understanding and assessment possibilities.
B - Echocardiography
Echocardiography is usually carried out in midpregnancy (1, 2, 3). Its performance in detection rates and specificity varies according to the ultrasound equipment used, examiner skills and experience, gestational age, fetal position and other factors that might limit visibility.
Ultrasound markers in the first trimester can be used for cardiovascular anomaly detection. Those are not specific for cardiovascular anomalies but are associated with much higher risk than that of the general population and fetal aneuploidies.
- Fetal aneuploidies: Screening for fetal aneuploidies (abnormal number of chromosomes) is now part of routine pregnancy care. It is best done from 11 to 14 weeks of pregnancy (8,9). Examination must look for major anomalies in the fetus, its heart rhythm, and check for markers in fetal aneuploidies. The markers associated with high risk for cardiovascular anomalies are (10, 11, 12): 1) Increased nuchal translucency 2) Tricuspid valve regurgitation 3) Reversed a-wave in ductus venosus (Fig. 2).
- In first trimester ductus venosus (DV) reversed a wave or tricuspid regurgitation in fetus are associated with increased risk for fetal cardiovascular anomaly (11,12) thus prompting for detailed asessment at 16-18 weeks instead of 22 weeks. Outflow tract stenosis can be detected and a velocity more that 100 cm/s is associated with development of hypoplastic leaft heart syndome (HLHS). Fetal valvuloplasty by baloon dilation in midpregnancy can result in succesful 2-ventricle circulation after birth (13, 14).
Fig 1.Three-dimensional rendering 13 weeks, spectacular for parents, apparently normal fetus.
On such images, the very rare pentalogy of Cantrell with ectopia cordis could be suspected (abdominal defect with displacement or eventration of the heart through the abdominothoracic wall).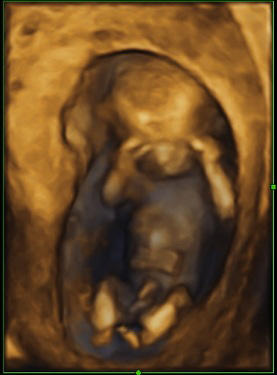
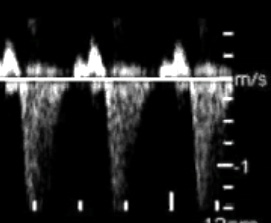
Fig.2 – The markers associated with high risk for cardiovascular anomies.; a.Increased nuchal translucency; b. Reversed a-wave in ductus venosus; c. Tricuspid valve regurgitation
2a. Increased nuchal translucency translucency is normal collection of fluid in the nuchal area; increased by more than 3 mm in embryonic and early fetal period raises risk for major cardiovascular anomalies to 6-15% depending on individual value; 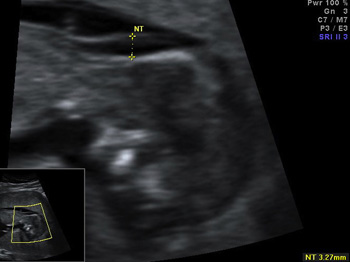 2b.Reversed a-wave in ductus venosus; d that was found at 11-14 weeks in TOF, AVSD, CoA, DORV, HLHS, TGA, Ebstein's anomaly, pulmonary stenosis and atresia, aortic stenosis;
2b.Reversed a-wave in ductus venosus; d that was found at 11-14 weeks in TOF, AVSD, CoA, DORV, HLHS, TGA, Ebstein's anomaly, pulmonary stenosis and atresia, aortic stenosis;
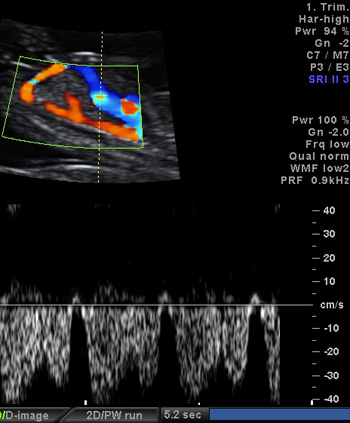
2c.Tricuspid valve regurgitation present in 30-35% of major cardiovascular anomalies in first trimester of pregnancy
Conclusions
- The burden of disease of congenital cardiovascular anomalies come mainly from low risk pregnancies.
- Low risk pregnancies benefit from first trimester ultrasound screening with detailed assessment as well as second trimester anomaly ultrasound screening. Those are ways for a low risk pregnancy to be reclassified as high risk and receive specialist care (prognosis and outcome might be influenced as a consequence).
- For high risk pregnancies fetal echocardiography should be offered as soon as in the first trimester or in mid-pregnancy.
- Trough this approach the vast majority of congenital cardiovascular disease will be diagnosed “in utero” and timely intervention with delivery in environment with specialised care for fetal affections will provide best chances for future generations.


 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.