First discovered in 1961 and leading up to the 70s, amiodarone was used for its antiarrhythmic properties, although not officially before the year 1985, when the United States finally approved its use. The Food and Drug Administration (FDA) had been reluctant to approve the drug due to initial reports showing an increased incidence of adverse pulmonary effects. European pharmaceutical companies began discussing with the institution in the mid-80s and threatened to suspend their (free) supply of the drug to American physicians, should the FDA not grant official approval. Authorisation was finally issued in December 1985, making it one of the few drugs approved by the FDA without prior randomised clinical trials.
I - Mechanism of action and pharmacologist effects
The two atoms of iodine contained in the amiodarone molecule, a benzofuran derivative, are essential to its antiarrhythmic properties. Using the Vaughan-Williams classification of antiarrhythmic drugs, amiodarone prolongs the Phase 3 action potential of cardiac cells and it also has all the Class 4 electrophysiological characteristics as well.
Absorption is extremely slow, and bioavailability is also low and variable. Latency between administration and effect, however, can be reduced by using a high loading of oral or intravenous dose.
On the other hand, the drug binds completely to plasma proteins and has a large distribution volume. It is then metabolised in the liver. Clearance is also low with an estimated elimination half-life of 30–180 days due to its metabolite, desethylamiodarone, which causes the drug to accumulate in peripheral tissues that act as reservoirs due to their affinity for intralisosomals phospholipids. These inclusion bodies have been found in the lungs, liver, heart, skin, corneal epithelium and peripheral nerves, which explain its toxic effects in many organs and the proportional relationship between toxicity, duration of use and cumulative dose.
A standard daily maintenance dose of 200 mg of amiodarone releases 75 mg of organic iodine. It is important to stress that normal daily requirement of iodine is 0.2 to 0.8 mg - more than 100 times lower that what is delivered with amiodarone.
Low-dose amiodarone (100-300 mg per day) is effective for a wide variety of ventricular and supraventricular arrhythmias, including as part of a rhythm control strategy for patients with atrial fibrillation, especially when structural heart disease is present. Where other drugs have failed to control arrhythmias, high doses of amiodarone will be required and produce an increase in the incidence of serious adverse side effects.
II - Impact of amiodarone therapy on survival in the clinical setting
Several side effects have been described over time, especially in cases of chronic use. It is estimated that the prevalence of adverse effects related to treatment with amiodarone is 15% the first year and 50% in cases of prolonged administration. In addition, 20% of patients require discontinued therapy due to effects such as liver damage, problems with thyroid function and chronic pulmonary squeal, which have been described in up to 1% per year of treatment, with cumulative damage of between 5% and 10%. Lung damage is the best known: it is produced after long exposure to amiodarone (2.3), but there also have been reports of acute toxicity.
Here are the most frequent, collateral effects of the drug:
a) Lung
Amiodarone-induced pulmonary toxicity (APT) is one of the most serious side effects of use. Incidence is approximately 5% when doses of 400 mg or more are used daily.
Interestingly, the atrial fibrillation follow-up investigation of rhythm management study (AFFIRM) found an increased incidence of pulmonary toxicity in patients with pre-existing pulmonary disease. However mortality from pulmonary causes and overall mortality were no higher among these patients, when compared with those without pre-existing pulmonary disease.
The mechanism of action of this pathology can be direct by releasing free radicals, or indirect from hypersensitivity of the patient. The risk factors associated with this adverse effect have not been well identified, but may be related to cumulative doses, daily doses above 400 mg, advanced age and underlying lung disease.
Amiodarone pulmonary toxicity can present in various ways: pneumonia interstitial disease, respiratory distress or solitary pulmonary masses. Clinical scenarios are variable, the most common symptoms being dyspnea and dry cough, but symptoms can also include fever, weight loss, hemoptysis and chest pain aggravated by breathing movements. Chest radiographs will show irregular or uneven bilateral interstitial infiltrate changes (Figures 1 and 2). Respiratory function tests show a restrictive pattern with decreased and diffuse total lung capacity. The mechanism of pulmonary toxicity has not been established, but also appears to be dose-related.
The diagnosis of APT is complicated by its nonspecific symptoms, clinical findings and results of imaging and laboratory tests, so it is considered an exclusion diagnosis. Differential diagnosis should be conducted with heart failure, pneumonia, pulmonary embolism, and neoplastic disease. Prognosis is favourable, with a low mortality rate, except for patients who develop respiratory distress, whose mortality approaches 50%. (3)
Treatment is based on drug discontinuation and administration of corticosteroids, which should be continued for several months despite cases of initially improved patient state, since the long half-life of amiodarone may result in a new exacerbation of symptoms. Prevention and early detection of this potential complication requires a chest radiograph obtained at baseline and annually thereafter.
b) Thyroid
Clinical evidence of hypothyroidism occurs in up to 20% of patients taking amiodarone. Amiodarone prevents peripheral conversion of T4 to T3 and alters thyroid function tests. The large amounts of iodine contained in amiodarone may explain these effects.
Hypothyroidism is easily managed with levothyroxine and is generally not a cause for discontinuing amiodarone. Hyperthyroidism occurs in 3% of patients in iodine-deficient areas, but in 20% in areas in areas where iodine is sufficient. It can be difficult to recognise because many of the typical adrenergically mediated signs are blocked by amiodarone.
In patients taking amiodarone, the first month of treatment revealed an increase in T4 (20%-40%), reverse T3 (20%) and TSH, and a decrease in T3, which tends to return to normal at three months.
In some cases, the recurrence of atrial fibrillation in patients with amiodarone maintenance therapy, required prompt evaluation to rule out amiodarone-induced hyperthyroidism.
The increased risk occurs in patients with abnormal baseline thyroid function (autoimmune disease, goiter) and the elderly. Women with positive antithyroid antibodies are seven times more at risk for this complication.
We recommend an analysis of TSH, T4 and T3 at treatment start, one month into treatment and every six months thereafter.
The diagnosis is presumed when there are increased levels of TSH and decreased T4 and T3. We wish to remind our readers that the increase in TSH during the initial three months after treatment starts with amiodarone is not a problem in itself because it can be a transient phenomenon - treatment with amiodarone can be continued provided levothyroxine is added. Triiodothyronine may also be added at times if TSH values have not decreased adequately. Management will then require the assistance of an experienced endocrinologist and discontinuation of amiodarone therapy.
In all patients, thyrotropin levels should be checked before initiating amiodarone therapy and every every months thereafter.
c) Other areas - gastrointestinal, liver, cardiac, ophthalmic, skin
Gastrointestinal disturbances such as nausea, vomiting, diarrhea, or anorexia are frequent (30%) at baseline. However, some of the most feared gastrointestinal side effects - hepatitis and liver cirrhosis - are rare (<3%). It is recommended to test liver function at initiation of treatment, and then every six months. At treatment start, an increase in liver enzymes is generally observed; treatment withdrawal is not recommended unless their values exceed two or three times normal. This elevation, according to certain authors, is not indicative of having to stop the drug: nevertheless, close monitoring is recommended.
Cardiac adverse effects including bradycardia have been reported in 5% of patients, and polymorphic ventricular arrhythmias (torsade de pointes) in 1%-2% of patients, especially among those subjects with risk factors for it. Therefore, an ECG should be performed at baseline and then annually. In patients with a cardioverter defibrillator (ICD), amiodarone can increase the cycle length of ventricular tachycardia, which can lead to them being detected and can interfere with the effectiveness of the device (4).
Finally, among other minor adverse effects, nearly 100% of patients receiving this drug for more than six months have cornea micro-deposits. Their presence does not require withdrawal of treatment since they are not related to impairment of visual acuity. Ocular reactions, such as optic neuritis, atrophy with loss of vision or both, are more serious, but they are rare and there is no proven causality of amiodarone with respect to these. Ophthalmologic evaluation is indicated only if there is a visual deficit.
Other reported side effects include photosensitivity and blue skin pigmentation, which disappear after discontinuation of treatment. In our patient, after completing various tests and suspecting that the patient's clinical picture corresponded to TPA, amiodarone was discontinued and corticosteroid therapy was initiated (40-60 mg daily dose for six months down). With this treatment, the patient experienced satisfactory clinical and radiological recovery.
Finally, amiodarone may cause hypotension in approximately 15% of patients receiving it intravenously. Hypotension can be treated initially by decreasing the infusion rate.
Figure 1A. Posteroanterior radiographs of the chest taken at the time of clinical presentation and after three months of treatment. Amiodarone infiltrates alveolointerstitial bilateral basal lung fields; however, media have disappeared at follow-up (image further below).
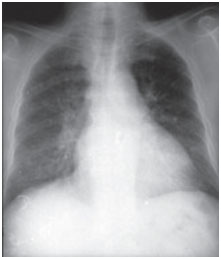
Figure 1B. Computed tomography of chest- in same patient, bilateral and diffuse alveolointerstitial infiltrated sample with air bronchogram areas.
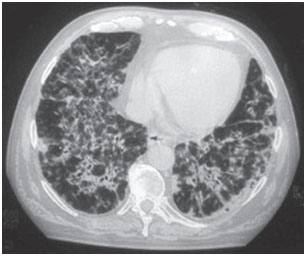
Table I summarises the most common side effects, diagnostic strategies and recommended follow-ups.




 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.