Metabolic syndrome clusters risk factors related to lipid metabolism: 1) high-density lipoprotein cholesterol and triglycerides, and 2) glucose metabolism (impaired glucose tolerance/diabetes). Moreover, metabolic syndrome includes 3) a hemodynamic parameter (blood pressure) and 4) an adiposity measurement (waist circumference).[1, 2, 3]
ESC guidelines on Dyslipidemias state that metabolic syndrome designates subjects who are at higher risk for cardiovascular disease, however how to capture this added risk, is a matter of debate. Moreover, following established threshold values, parameters of metabolic syndrome are considered present or not - in a categorical manner. This pragmatic approach does not factor in the dynamic behaviour of most of the variables, which flux according to external, environmental, circadian and/or postprandial factors. Thus, chances are that one or more metabolic syndrome component(s) may at times lead to high diagnostic values, while being normal at other readings, causing divergent diagnoses and variable estimation of cardiovascular risk in a particular subject. [4]
While it is true that the clinical setting offers well established methods to evaluate dynamic patterns of blood pressure and glucose metabolism, with ambulatory blood pressure monitoring and OGTT (oral glucose tolerance test) - having enabled the definition of pre-hypertension and pre-diabetes [5, 6], dynamic behavior of triglycerides on the other hand has not been established to date as measurable in the clinical setting, mainly due to lack of standardisation in loading tests and variability in the correlations between baseline and after loading values. [7] However, dynamic postprandial lipaemia has been recognised as a factor increasing cardiovascular risk.[8] Indeed, postprandial hypertriglyceridemia is considered a robust risk factor of cardiovascular disease, independent from waist circumference, glycated haemoglobin, age, HDL-c and ethanol consumption. [9, 10]
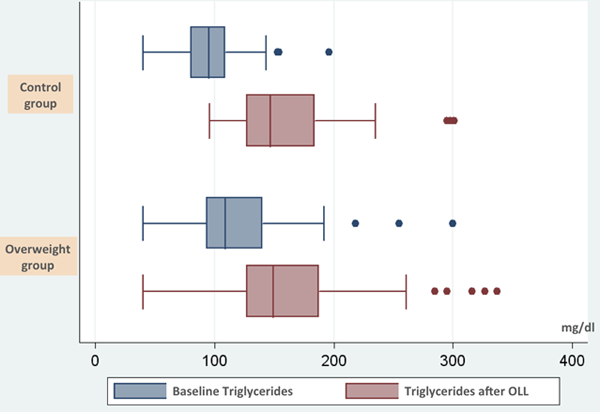
We correlated response in dynamic postprandial lipaemia from a systematic oral lipid loading test (SOLLT) - 400 ml of whole milk and a piece of standard pastry (85 g), a relatively modest lipid intake, with the parameters that conform to the definition of the metabolic syndrome - adiposity indexes in particular [11]. Glucose, and blood pressure measurements, as well as triglycerides were performed baseline, 2 and 4 hours after the loading test. Responses were interpreted according to a Consensus Group that established a normal postpandrial response to be under 180 mg/dl following free food intake and 220 mg/dl after fixed loading test. [7] Patients with a body mass index above 25 kg/m2 from one group exhibited a greater triglyceride increase (64%) compared to healthy individuals from the normal control group (a 54% increase). A difference from baseline was noted as well and other dynamic measurements, systolic and diastolic blood pressure, capillary glucose after glucose tolerance test were also higher in the high body mass index group (Fig. 1)
In healthy individuals with normal glucose and lipid metabolism, maximal triglycerides values achieved were related to three of the clinical variables used in the present study: weight, waist circumference (cm) and body mass index (BMI) (Table 2).
Triglycerides values after oral lipid loading test offered better correlation coefficients than baseline values with body mass index, waist circumference and baseline diastolic blood pressure. However, it appears that besides its atherogenic and prothrombotic components, abdominal fat is responsible for the abnormal clearance of triglycerides after lipid intake, despite baseline levels that are within normal limits. [12]
It is noteworthy that, despite the fact that the only difference controlled at baseline between the two groups was body mass index or increased waist circumference, both groups differed also in their blood pressure, capillary glucose and triglycerides baseline levels. Even though the readings do not surpass normal range limits of metabolic syndrome, they are closer to the upper limit than those from the control group with normal body mass index. Thus, the hypothesis that this particular population is at increased risk for developing further overt metabolic syndrome is likely. Furthermore, study of triglycerides behavior in this population have lead to this finding: the overweight individuals had significantly higher triglycerides both at baseline and after SOLLT than subjects from the control group. This result might have been predicted from intuiton only, but might also confirm the convenience of the lipid loading test to unmask underlying metabolic initial disorder.
In clinical practice, the proposed and validated SOLLT could be used to reveal the underlying “subclinical” components of a subsequent metabolic syndrome in individuals that comprise the main feature of it, abdominal adiposity, or genetic factors making an individual vulnerable to it. [13] This test could fit in within other preventive strategies such as physical exercise, particular dietary [14, 15] or pharmacological products [16, 17] to improve the various features of the metabolic syndrome, and postprandial dyslipidemia specifically. Despite the lack of studies examining improvement of postprandial response following preventive strategies, this might be a feasible and useful tool for lipid loading in populations at risk for metabolic syndrome.
Conclusion:
Lipid loading test is considered a research metabolic tool with little clinical application. Capillary triglycerides measurement is a simple clinical measurement that might be useful for clinicians to precisely define lipid metabolic impairment in its early stages.
Table 1. Glucose, blood pressure and triglycerides after oral lipid loading in the cohort of overweight patients.
| Variable | After OGTT | After OLL |
|---|---|---|
| Systolic blood pressure (mm Hg) | 126 (20) | 135 (17) |
| Diastolic blood pressure (mm Hg) | 81 (13) | 87 (11) |
| Glucose (mg/dl) | 152 (33) | 121 (23) |
| Triglycerides (mg/dl) | 109 (89-141) | 183 (156-209) |
Values represent mean (standard deviation) except triglycerides: median (interquartile rank).
Abbreviations: OGTT: oral glucose tolerance test; OLL: oral lipid loading.
Table 2. Correlation between maximal triglycerides values after oral lipid loading with several clinical variables.
| Variable | Correlation Coefficient* | p value |
|---|---|---|
| Age | -0,028 | 0,847 |
| Sex | -0,036 | 0,806 |
| Weight | 0,317 | 0,025 |
| Waist circumference | 0,299 | 0,035 |
| Systolic BP | 0,230 | 0,108 |
| Diastolic BP | 0,234 | 0,102 |
| Glucose | 0,075 | 0,605 |
| Body mass index (BMI) | 0,326 | 0,021 |
*Pearson
Abbreviation: BP: blood pressure.
Figure 1. Median and interquartile rank of baseline triglycerides and after lipid loading test in the control group (n= 50) and overweight group (n= 68).
Abbreviations: OLL: oral lipid loading.


 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.