Background
Thus far, biological prosthetic valves would classically be categorised as stented or stentless, according to whether the biological tissue used to make the leaflets - aortic valve of the pig or bovine/equine pericardium - is sewn into a supporting frame (stent) or not. Whether stented or stentless, prostheses were fixed to the native aortic annulus with individual surgical sutures, with or without pledgets or as a continuous suture approximating the cuff of the prosthesis to the native annulus. Sutures were made of non-resorbable material functioning to immobilise the prosthesis and to seal the contour of the valve in the left ventricular outflow tract (LVOT) in order to avoid paravalvular leaks (figure 1).
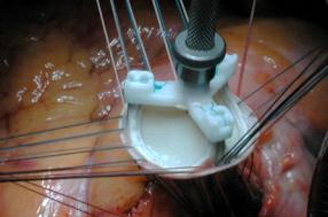
Figure 1. Conventional aortic valve replacement: This stented bioprothesis is fixed in place with several interrupted sutures.
With the development of transcatheter aortic-valve implantation (TAVI), prostheses have been implanted without surgical fixation. The radial pressure of the stent suffices to fixate the valve in the LVOT- the stent is balloon expandable in the case of the Sapien valve; and nitinol when using the CoreValve prosthesis.
Although valve migration and embolisation are infrequent, paravalvular leaks remain an important drawback of TAVI valves. (1) Paravalvular leakage has been attributed to a number of possible causes- the retained calcified native valve possibly being the most important.
In the last two years, surgical prostheses have been developed to incorporate sutureless fixation. Thus, in an arrested heart, the aorta is opened, the diseased aortic valve is completely excised and the aortic annulus is prepared as in conventional surgery. The size of the annulus is measured by adapted calibrators to determine the prosthesis to use. The appropriate valve is then implanted into the aortic annulus under direct visual control and is maintained in-situ by the radial force of its stent- no sutures required. Depending on the model, a few sutures may be employed to guide the descent of the prosthesis or eventually tied to further consolidate annular fixation.
The advantages of these new generation sutureless valves combine those of open surgical aortic valve replacement (AVR) and the facility of TAVI. They include:
- Complete excision of the diseased valve
- Anatomical tailoring to individual patient anatomy
- Atraumatic introduction with minimal or no crimping of the valve leaflets allowing more predictable long-term outcomes
- Valves are self-anchoring (no need for sutures), self-expanding for easy implantation and good visibility
- Shorter cardiopulmonary bypass
- Permits minimally invasive cardiac surgery procedures while delivering gold-standard surgical outcome.
These goals have been achieved by a number of sutureless or (near sutureless) prosthesis currently available:
A - 3f Enable valve ™
This was the first to be approved. It is based on the ATS 3f® Aortic Bioprosthesis and a self-expanding Nitinol™ frame to hold the valve in its position (figure 2). Foldable into a small diameter, it can be implanted through a minimally invasive technique such as ministernotomy or thoracotomy. It has three equine pericardial leaflets giving it a tubular design that allows for decreased leaflet stress and preservation of aortic sinuses (2,3).
A multicenter clinical study evaluated the safety and efficacy of this bioprosthesis in 140 patients undergoing isolated aortic valve replacement with or without concomitant procedures. Mean systolic gradient was 9.04 ± 3.56 and 8.62 ± 3.16 mm Hg with mean effective orifice area of 1.69 ± 0.52 and 1.67 ± 0.44 at 6 months and 1 year, respectively.
Early complications included three major paravalvular leaks (PVL; 2.1%) resulting in valve explantation and one thrombo-embolic (0.7%) event. Three late adverse events occurred (2.5% per patient-year): one due to PVL and two due to endocarditis. There was an additional case of late endocarditis (0.8% per patient-year) that resolved with medical management. No structural deterioration, valve-related thrombosis or hemolysis was documented in the total accumulated follow-up of 121.8 patient-years. (4)
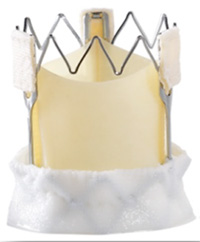
Figure 2. The 3f Enable aortic bioprosthesis (Medtronic, Minneapolis, MN)
B - Perceval S ™
Perceval S (figure 3) is self-anchoring and self-expanding, made of bovine pericardium and mounted on an elastic alloy frame. Sinusoidal struts (structural element) anchor the device in the Valsalva sinus and annular sealing is obtained with brief low-pressure balloon dilation.
A European, multicenter, prospective, non-randomised trial evaluated its feasibility in 30 patients. The mean aortic cross-clamp and ECC times were 34 +/- 15 min and 59 +/- 21 min, respectively. There was one in-hospital death (3.3%), and three within 12 months of follow up (one valve-related, and two independent of the valve implantation). A total of 28 patients were assessed at one month post-implantation, and 23 after 12 months. No migration or dislodgement of the valve occurred, but there were two mild paravalvular leakages and two mild intravalvular insufficiencies.(5) In a follow up study, aortic cross-clamp time needed for aortic valve replacement was 18 ± 6 minutes. This was associated with excellent early clinical and hemodynamic outcome in high-risk patients. (6)
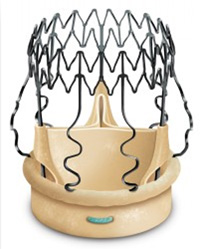
Figure 3. The Perceval S Sutureless biological valve (Sorin Group, Saluggia, Italy)
C - INTUITY Valve System ™
The valve system is based on the Carpentier-Edwards PERIMOUNT Magna Ease aortic valve producing a stented tri-leaflet bovine pericardial bioprosthesis (the Magna valve platform) with a balloon expandable, cloth-covered stent frame at the inflow aspect (figure 4). The valve is positioned supra-annularly using three guiding sutures. The sutures are tied, and the aortotomy closed following frame expansion with a balloon catheter. Data from the TRITON study (7), a multi-center European prospective study in 90 patients with severe, symptomatic aortic stenosis or stenosis-insufficiency evaluating the feasibility, safety and performance of the EDWARDS INTUITY Valve System, showed a technical success rate of 94 percent in an isolated AVR or AVR with a concomitant procedure. For the isolated AVR procedures, mean aortic cross-clamp times were reduced by 43 percent, and mean bypass times by 41 percent, compared to the STS National Database.
In the initial cohort of 62 patients, at 3 months, trace, mild and moderate Paravalvular leaks were observed in 2, 1 and 1 patient, respectively (n=23). Mean systolic Pressure Gradient was 7.4 ± 3.3 (n=21) mm Hg. (8)

Figure 4. INTUITY Valve System (Edwards Lifesciences Corporation, Irvine, CA)
Conclusion:
The sutureless valve implantation technique is feasible and safe. Valve implantation has resulted in excellent hemodynamics and significant clinical improvement. Shortening the aortic cross-clamp and ECC times may help to reduce mortality and morbidity. However, long term follow-up is necessary to confirm the clinical advantage of these prostheses.
Declaration of Interest: None declared.


 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.