Background
Despite many advances in its treatment over the past decades, heart failure remains a problem of high prevalence, morbidity and mortality worldwide (1). Since its appearance in the 80's (2) and its clinical application in 90 years(3), cardiac resynchronisation therapy (CRT) has become an essential therapeutic tool in the treatment of heart failure patient today. Its aim is to restore electrical synchrony, commonly impaired in these patients, and thus the cardiac function. Its results have been excellent. According to a recent meta-analysis of McAlister et al (4), CRT provides significant improvement in functional class, left ventricular ejection fraction (LVEF), the distance walked at 6 minutes, quality of life and a reduction in hospitalisation for heart failure and overall mortality, mainly due to a reduction in mortality from progressive heart failure. Moreover, these benefits occur in a stable and progressive manner. However, there are still 20-30% of patients who do not respond to therapy (5). Therefore, it is essential to make a careful selection of candidates. Several randomised trials have suppported its recommendation in patients in sinus rhythm, NYHA functional class III-IV, left ventricular ejection fraction (LVEF ) ≤ 35% and QRS ≥ 120 ms (1,6,7,8) . Subsequent studies have recommended the use of CRT in patients ins sinus rhythm, NYHA functional class II, LVEF ≤ 30% and QRS ≥ 130 ms (9) (figure 1). While these indications are clear (Recommendation Class I, Level A), there is less consensus about various sub-populations underrepresented in clinical trials.
Figure 1. Recommendations for the use of CRT where the evidence is strong from the 2012 guidelines for the diagnosis and treatment of acute and chronic heart failure (9).
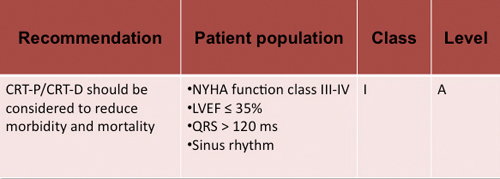
Figure legend:Class I recommendation. CRT-P: Cardiac resynchronization device. CRT-D device for cardiac resynchronization and defibrillation. HF: heart failure. SR: Sinus rhythm. LBBB: left bundle branch block. LVEF: Left ventricular ejection fraction. *:For patients who are expected to survive with good functional status for > 1 year. **: Despite optimal pharmacological therapy.
I - Pathophysiology of disorders of intraventricular conduction
Patients with heart failure on the other hand often present intraventricular conduction disorders. The most common is left bundle branch block (LBBB, 15-47%), followed by right bundle branch block (4-25%) and, less frequently, non-specific intraventricular conduction delay (IVCD) (10). These disorders result in a specific alteration in the pattern of electrical activation that impairs the sequence of ventricular contraction in a different manner for each of these disorders.
A) Left bundle branch block
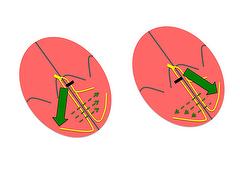
Activation of the interventricular septum and right ventricle in left bundle branch block (LBBB) occurs at an early stage through the right branch. As a result, the left ventricle is activated from the septoapical to the posterolateral region and from the endocardium to the epicardium (figure 2) in a slow manner because of the absence of conduction tissue (cell to cell conduction). Together, these particularities lead to uncoordinated and ineffective myocardial contraction. On the one hand, early septal contraction does not increase pressure in the left ventricular cavity due to the absence of opposition from the posterolateral wall. Subsequently, lateral contraction occurs during late systole so that the intraventricular maximum pressure is reached at the beginning of diastole. The result is a reduction in stroke volume that, together with mitral regurgitation secondary to late activation of papillary muscle, causes a significant decrease in cardiac output.
The purpose of cardiac resynchronisation therapy in these patients is to eliminate this delay in activation by simultaneous stimulation of the septum and left ventricular wall, and thus, allowing for more efficient mechanical contraction.
Figure 2. Pathophysiology of intraventricular conduction disorders. On the left side, ventricular myocardial activation vector in patients with LBBB. On the right, ventricular myocardial activation vector in patients with RBBB.
B) Right bundle branch block
However, other pathophysiological reasons such as the possibility of an additional delay in left ventricular activation might explain a beneficial response in these patients:
- Fantoni et al (11) prospectively evaluated the endocardial activation pattern in 100 patients with heart failure, RBBB and an indication for cardiac resynchronisation therapy. By performing activation maps with an electroanatomic navigation system, they documented two major findings: 1) RBBB patients showed a delay in activation of the anterior and lateral walls of the right ventricle similar to that displayed by LBBB patients in the left ventricle. In addition, 2) RBBB patients had a pattern of activation and conduction delay in left ventricle similar to that of patients with LBBB.
Alhough this study described a very limited sample (only 6 of the 100 patients had RBBB), the possibility of an additional delay in left ventricular activation in patients with RBBB and left ventricular dysfunction has also been reported in other studies. - Richman and Wolff (12) used the term “LBBB masquerading as RBBB” to illustrate this concept in classical electrocardiographic studies. They described patients who showed a pattern of RBBB in precordial leads but a LBBB pattern in limb leads and suggested the possibility of an additional delay in left ventricular activation.
- Most recently, echocardiographic studies have also shown the frequent presence of left ventricular asynchrony in patients with RBBB and left ventricular dysfunction (13), especially in patients with ischemic heart disease, a well-known factor of delayed left ventricular activation in patients with LBBB (14), and particularly in presence of septal and inferior myocardial infarctions that could alter the electrocardiographic pattern.
- Experimental studies have also led to similar conclusions. Byrne et al (15) developed an experimental canine model in which tachycardiomyopathy and right or left bundle branch block by radiofrequency ablation were induced. They noted that while the widening of the QRS and impaired LVEF were similar in both groups (RBBB and LBBB), in the group with isolated RBBB, mechanical dyssynchrony and improvement in response to CRT were lower and the response was even more favourable with right ventricular pacing.
According to these results, it has been suggested that some patients with RBBB and delayed left ventricular activation may benefit from biventricular pacing.
Additionally, several options have been offered to help detect left ventricular delay, and thus appropriate patients for CRT therapy: - Garrigue et al (16) prospectively studied 12 patients with NYHA functional class II-III, LVEF <35% and QRS longer than 140 ms. All patients were implanted with a biventricular pacemaker and were followed for one year. The result was an improvement from a clinical (significant improvement in functional class and exercise capacity), electrocardiographic (significant narrowing of the QRS) and echocardiographic (significant reduction of diastolic diameter and septolateral dyssynchrony and improvement of the peak aortic velocity and mitral regurgitation) point of view that only occurred in the 9 patients with significant left ventricular mechanical dyssynchrony.
- While mechanical dyssynchrony could be the parameter that allowed us to select those patients with RBBB who would benefit from cardiac resynchronisation therapy, the limited sensitivity, specificity and reproducibility of conventional echocardiographic parameters of mechanical dyssynchrony observed in the PROSPECT trial (17) would not support this method. It is possible that the use of more advanced techniques such as strain rate, speckle tracking and three-dimensional echocardiography, may change these results in the near future.
- The electrocardiographic assessment of the presence of additional involvement of any of the fascicles of the left bundle branch has also been suggested to detect left ventricular conduction delay. Hemifascicular blocks are a common disorder in patients with heart failure and RBBB. A subanalysis of MIRACLE trial (18), reported that included patients with RBBB associated anterior hemiblock was 56% of cases and posterior hemiblock 26% of cases. It also documented that a significant percentage of patients with anterior hemiblock (48%), especially if the left axis deviation is greater than -30 °, have mechanical dyssynchrony that can sometimes be even comparable to that of LBBB (19).
- Attending to these considerations, Chandra et al (20) carried out a study to compare the effect of cardiac resynchronisation therapy in patients with isolated RBBB versus patients with RBBB and left fascicular block. Of 271 patients enrolled, 44 had RBBB, 18 had isolated RBBB and 26 with involvement of some left hemifascicle: 18 anterior hemiblock (LAHB) and 8 posterior hemiblock (LPHB). The primary endpoint was to evaluate the evolution of functional class and LVEF. No patient with isolated RBBB showed improvement in functional class compared with 7 of 26 of the group with associated hemiblock. Regarding LVEF, there was a significant increase in 4 patients with isolated RBBB group and 18 patients with associated hemiblock, and only in this group was the increase in LVEF significant.
However, although the hypothesis of delayed left ventricular activation as a selection criterion is very attractive, there have been reports of patients in whom biventricular pacing, despite the existence of basal intraventricular asynchrony, has caused a worsening of the asynchrony and serious functional impairment of the patient (20). It is possible that other factors such as underlying heart disease and location of the area with mechanical delay also condition the result. That is, the asynchrony may not be similar in subjects with or without myocardial scar areas or left, right or biventricular dysfunction.
Conclusion:
With respect to the pathophysiolgic basis of cardiac resinchronisation therapy in patients with advanced heart failure and right bundle branch block, we should consider that:
- The pathophysiological effect of biventricular pacing in patients with RBBB is not clear.
- The ECG of patients with heart failure can be influenced by the underlying heart disease and therefore not correspond with the real pattern of ventricular activation.
- Experimental, electrocardiographic, echocardiographic and electrophysiologic studies show that patients with RBBB may have an additional delay in left ventricular activation because of the pathological substrate of the associated heart disease. Therefore, they could benefit from CRT.
- A history of ischemic heart disease and delayed mechanical or electrical activation could serve as discriminators, although there are no studies relevant enough to support this attitude.
- It is possible that other factors such as underlying heart disease and location of the area with mechanical delay also condition the result.
- Nevertheless, in the absence of adequate evidence in this regard, it should be considered that some patients might not respond or even suffer a worsening of asynchrony with clinically significant impact.


 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.